Keith Morse
MedHELM: Holistic Evaluation of Large Language Models for Medical Tasks
May 26, 2025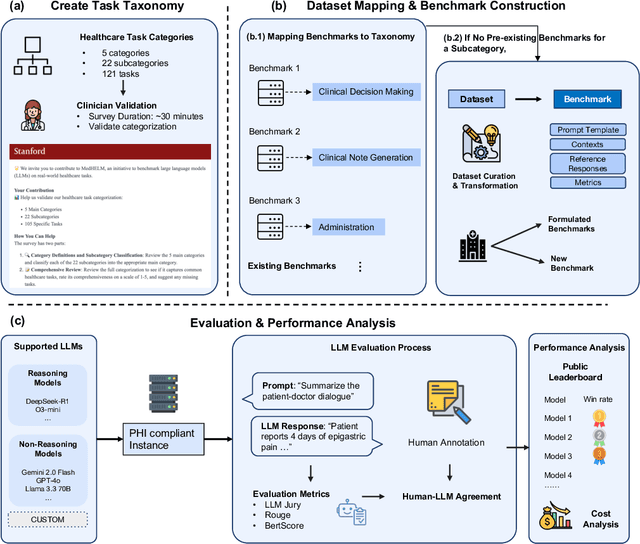
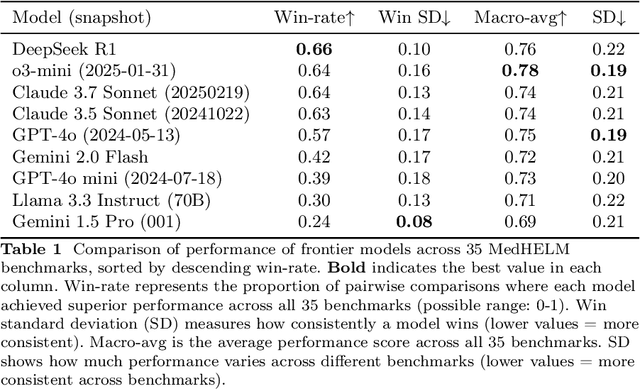
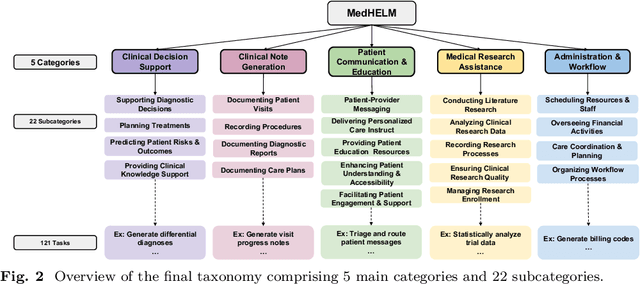

Abstract:While large language models (LLMs) achieve near-perfect scores on medical licensing exams, these evaluations inadequately reflect the complexity and diversity of real-world clinical practice. We introduce MedHELM, an extensible evaluation framework for assessing LLM performance for medical tasks with three key contributions. First, a clinician-validated taxonomy spanning 5 categories, 22 subcategories, and 121 tasks developed with 29 clinicians. Second, a comprehensive benchmark suite comprising 35 benchmarks (17 existing, 18 newly formulated) providing complete coverage of all categories and subcategories in the taxonomy. Third, a systematic comparison of LLMs with improved evaluation methods (using an LLM-jury) and a cost-performance analysis. Evaluation of 9 frontier LLMs, using the 35 benchmarks, revealed significant performance variation. Advanced reasoning models (DeepSeek R1: 66% win-rate; o3-mini: 64% win-rate) demonstrated superior performance, though Claude 3.5 Sonnet achieved comparable results at 40% lower estimated computational cost. On a normalized accuracy scale (0-1), most models performed strongly in Clinical Note Generation (0.73-0.85) and Patient Communication & Education (0.78-0.83), moderately in Medical Research Assistance (0.65-0.75), and generally lower in Clinical Decision Support (0.56-0.72) and Administration & Workflow (0.53-0.63). Our LLM-jury evaluation method achieved good agreement with clinician ratings (ICC = 0.47), surpassing both average clinician-clinician agreement (ICC = 0.43) and automated baselines including ROUGE-L (0.36) and BERTScore-F1 (0.44). Claude 3.5 Sonnet achieved comparable performance to top models at lower estimated cost. These findings highlight the importance of real-world, task-specific evaluation for medical use of LLMs and provides an open source framework to enable this.
Standing on FURM ground -- A framework for evaluating Fair, Useful, and Reliable AI Models in healthcare systems
Mar 14, 2024



Abstract:The impact of using artificial intelligence (AI) to guide patient care or operational processes is an interplay of the AI model's output, the decision-making protocol based on that output, and the capacity of the stakeholders involved to take the necessary subsequent action. Estimating the effects of this interplay before deployment, and studying it in real time afterwards, are essential to bridge the chasm between AI model development and achievable benefit. To accomplish this, the Data Science team at Stanford Health Care has developed a Testing and Evaluation (T&E) mechanism to identify fair, useful and reliable AI models (FURM) by conducting an ethical review to identify potential value mismatches, simulations to estimate usefulness, financial projections to assess sustainability, as well as analyses to determine IT feasibility, design a deployment strategy, and recommend a prospective monitoring and evaluation plan. We report on FURM assessments done to evaluate six AI guided solutions for potential adoption, spanning clinical and operational settings, each with the potential to impact from several dozen to tens of thousands of patients each year. We describe the assessment process, summarize the six assessments, and share our framework to enable others to conduct similar assessments. Of the six solutions we assessed, two have moved into a planning and implementation phase. Our novel contributions - usefulness estimates by simulation, financial projections to quantify sustainability, and a process to do ethical assessments - as well as their underlying methods and open source tools, are available for other healthcare systems to conduct actionable evaluations of candidate AI solutions.
A Multi-Center Study on the Adaptability of a Shared Foundation Model for Electronic Health Records
Nov 20, 2023Abstract:Foundation models hold promise for transforming AI in healthcare by providing modular components that are easily adaptable to downstream healthcare tasks, making AI development more scalable and cost-effective. Structured EHR foundation models, trained on coded medical records from millions of patients, demonstrated benefits including increased performance with fewer training labels, and improved robustness to distribution shifts. However, questions remain on the feasibility of sharing these models across different hospitals and their performance for local task adaptation. This multi-center study examined the adaptability of a recently released structured EHR foundation model ($FM_{SM}$), trained on longitudinal medical record data from 2.57M Stanford Medicine patients. Experiments were conducted using EHR data at The Hospital for Sick Children and MIMIC-IV. We assessed both adaptability via continued pretraining on local data, and task adaptability compared to baselines of training models from scratch at each site, including a local foundation model. We evaluated the performance of these models on 8 clinical prediction tasks. In both datasets, adapting the off-the-shelf $FM_{SM}$ matched the performance of GBM models locally trained on all data while providing a 13% improvement in settings with few task-specific training labels. With continued pretraining on local data, label efficiency substantially improved, such that $FM_{SM}$ required fewer than 1% of training examples to match the fully trained GBM's performance. Continued pretraining was also 60 to 90% more sample-efficient than training local foundation models from scratch. Our findings show that adapting shared EHR foundation models across hospitals provides improved prediction performance at less cost, underscoring the utility of base foundation models as modular components to streamline the development of healthcare AI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge