Junbo Peng
Image-Domain Material Decomposition for Dual-energy CT using Unsupervised Learning with Data-fidelity Loss
Nov 17, 2023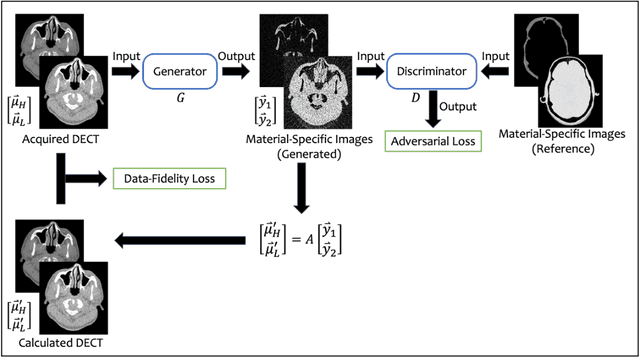
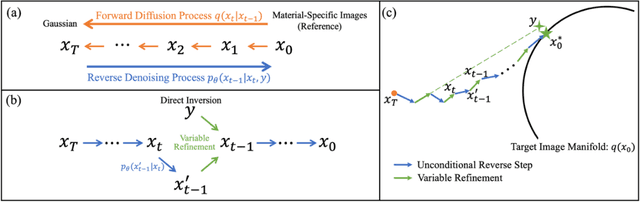
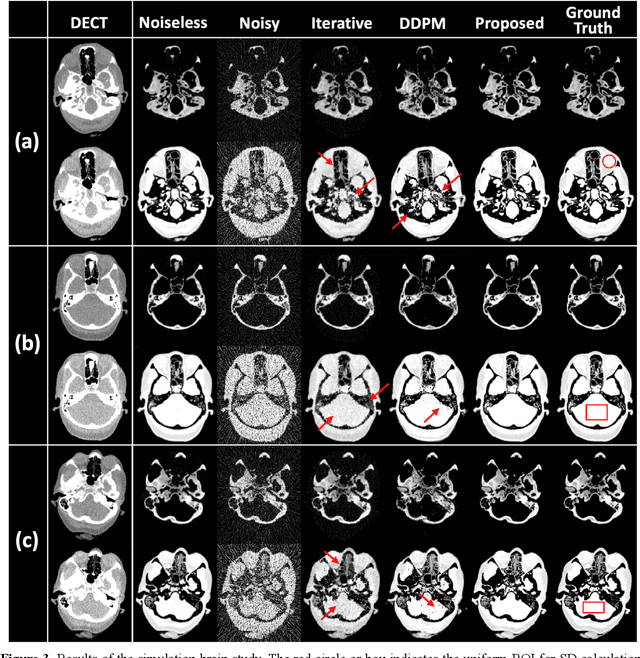
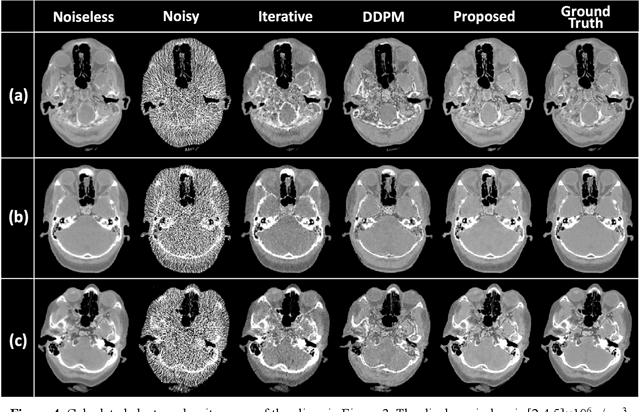
Abstract:Background: Dual-energy CT (DECT) and material decomposition play vital roles in quantitative medical imaging. However, the decomposition process may suffer from significant noise amplification, leading to severely degraded image signal-to-noise ratios (SNRs). While existing iterative algorithms perform noise suppression using different image priors, these heuristic image priors cannot accurately represent the features of the target image manifold. Although deep learning-based decomposition methods have been reported, these methods are in the supervised-learning framework requiring paired data for training, which is not readily available in clinical settings. Purpose: This work aims to develop an unsupervised-learning framework with data-measurement consistency for image-domain material decomposition in DECT.
Full-dose PET Synthesis from Low-dose PET Using High-efficiency Diffusion Denoising Probabilistic Model
Aug 24, 2023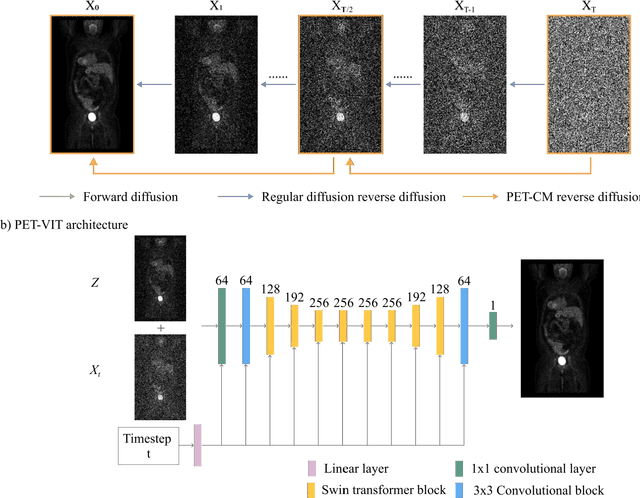


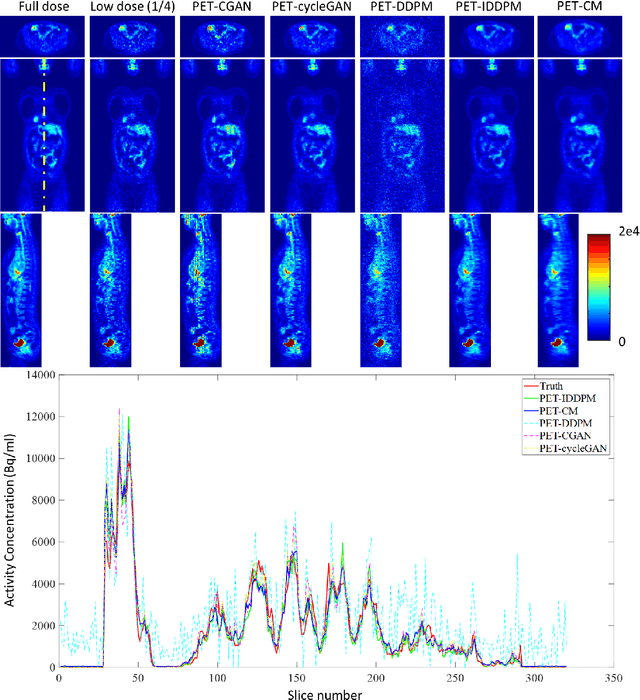
Abstract:To reduce the risks associated with ionizing radiation, a reduction of radiation exposure in PET imaging is needed. However, this leads to a detrimental effect on image contrast and quantification. High-quality PET images synthesized from low-dose data offer a solution to reduce radiation exposure. We introduce a diffusion-model-based approach for estimating full-dose PET images from low-dose ones: the PET Consistency Model (PET-CM) yielding synthetic quality comparable to state-of-the-art diffusion-based synthesis models, but with greater efficiency. There are two steps: a forward process that adds Gaussian noise to a full dose PET image at multiple timesteps, and a reverse diffusion process that employs a PET Shifted-window Vision Transformer (PET-VIT) network to learn the denoising procedure conditioned on the corresponding low-dose PETs. In PET-CM, the reverse process learns a consistency function for direct denoising of Gaussian noise to a clean full-dose PET. We evaluated the PET-CM in generating full-dose images using only 1/8 and 1/4 of the standard PET dose. Comparing 1/8 dose to full-dose images, PET-CM demonstrated impressive performance with normalized mean absolute error (NMAE) of 1.233+/-0.131%, peak signal-to-noise ratio (PSNR) of 33.915+/-0.933dB, structural similarity index (SSIM) of 0.964+/-0.009, and normalized cross-correlation (NCC) of 0.968+/-0.011, with an average generation time of 62 seconds per patient. This is a significant improvement compared to the state-of-the-art diffusion-based model with PET-CM reaching this result 12x faster. In the 1/4 dose to full-dose image experiments, PET-CM is also competitive, achieving an NMAE 1.058+/-0.092%, PSNR of 35.548+/-0.805dB, SSIM of 0.978+/-0.005, and NCC 0.981+/-0.007 The results indicate promising low-dose PET image quality improvements for clinical applications.
Synthetic CT Generation from MRI using 3D Transformer-based Denoising Diffusion Model
May 31, 2023


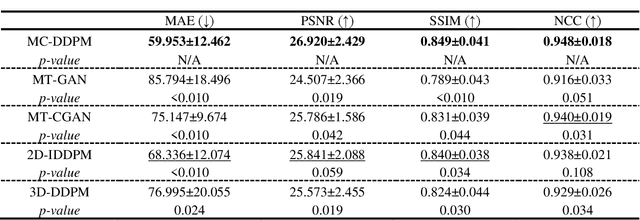
Abstract:Magnetic resonance imaging (MRI)-based synthetic computed tomography (sCT) simplifies radiation therapy treatment planning by eliminating the need for CT simulation and error-prone image registration, ultimately reducing patient radiation dose and setup uncertainty. We propose an MRI-to-CT transformer-based denoising diffusion probabilistic model (MC-DDPM) to transform MRI into high-quality sCT to facilitate radiation treatment planning. MC-DDPM implements diffusion processes with a shifted-window transformer network to generate sCT from MRI. The proposed model consists of two processes: a forward process which adds Gaussian noise to real CT scans, and a reverse process in which a shifted-window transformer V-net (Swin-Vnet) denoises the noisy CT scans conditioned on the MRI from the same patient to produce noise-free CT scans. With an optimally trained Swin-Vnet, the reverse diffusion process was used to generate sCT scans matching MRI anatomy. We evaluated the proposed method by generating sCT from MRI on a brain dataset and a prostate dataset. Qualitative evaluation was performed using the mean absolute error (MAE) of Hounsfield unit (HU), peak signal to noise ratio (PSNR), multi-scale Structure Similarity index (MS-SSIM) and normalized cross correlation (NCC) indexes between ground truth CTs and sCTs. MC-DDPM generated brain sCTs with state-of-the-art quantitative results with MAE 43.317 HU, PSNR 27.046 dB, SSIM 0.965, and NCC 0.983. For the prostate dataset, MC-DDPM achieved MAE 59.953 HU, PSNR 26.920 dB, SSIM 0.849, and NCC 0.948. In conclusion, we have developed and validated a novel approach for generating CT images from routine MRIs using a transformer-based DDPM. This model effectively captures the complex relationship between CT and MRI images, allowing for robust and high-quality synthetic CT (sCT) images to be generated in minutes.
Cycle-guided Denoising Diffusion Probability Model for 3D Cross-modality MRI Synthesis
Apr 28, 2023Abstract:This study aims to develop a novel Cycle-guided Denoising Diffusion Probability Model (CG-DDPM) for cross-modality MRI synthesis. The CG-DDPM deploys two DDPMs that condition each other to generate synthetic images from two different MRI pulse sequences. The two DDPMs exchange random latent noise in the reverse processes, which helps to regularize both DDPMs and generate matching images in two modalities. This improves image-to-image translation ac-curacy. We evaluated the CG-DDPM quantitatively using mean absolute error (MAE), multi-scale structural similarity index measure (MSSIM), and peak sig-nal-to-noise ratio (PSNR), as well as the network synthesis consistency, on the BraTS2020 dataset. Our proposed method showed high accuracy and reliable consistency for MRI synthesis. In addition, we compared the CG-DDPM with several other state-of-the-art networks and demonstrated statistically significant improvements in the image quality of synthetic MRIs. The proposed method enhances the capability of current multimodal MRI synthesis approaches, which could contribute to more accurate diagnosis and better treatment planning for patients by synthesizing additional MRI modalities.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge