Jose Bernal
Standardized Evaluation of Automatic Methods for Perivascular Spaces Segmentation in MRI -- MICCAI 2024 Challenge Results
Dec 20, 2025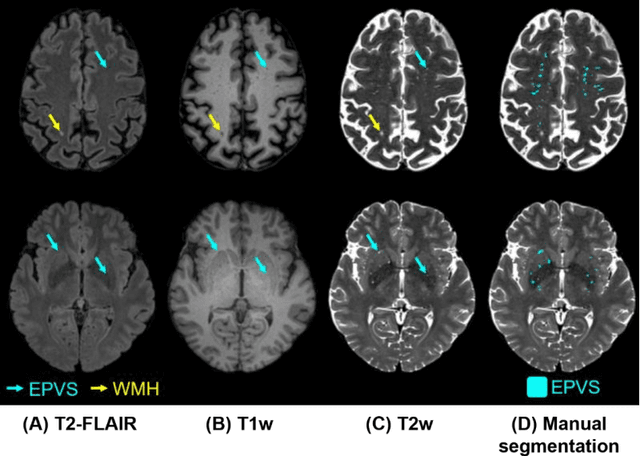
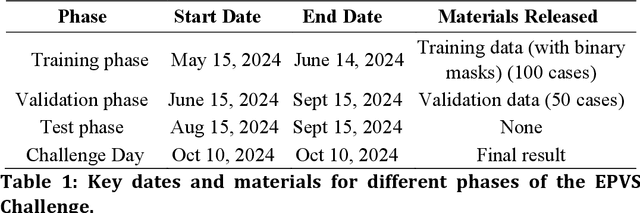
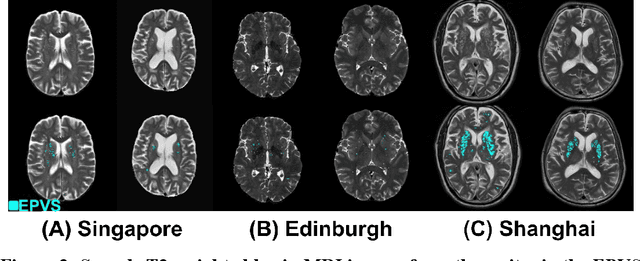
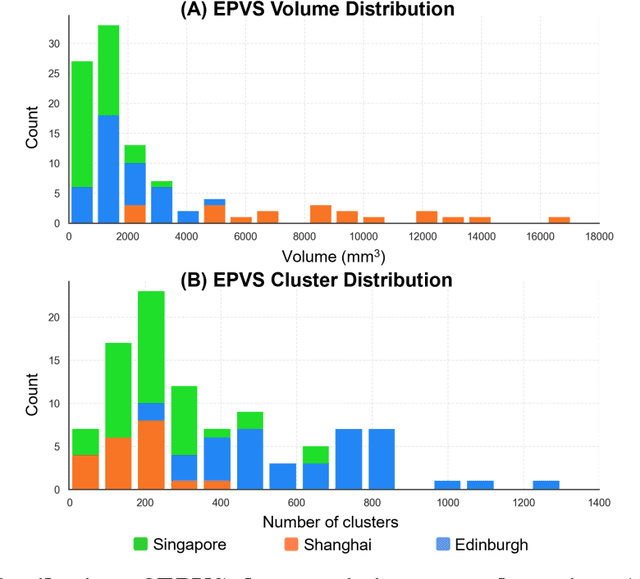
Abstract:Perivascular spaces (PVS), when abnormally enlarged and visible in magnetic resonance imaging (MRI) structural sequences, are important imaging markers of cerebral small vessel disease and potential indicators of neurodegenerative conditions. Despite their clinical significance, automatic enlarged PVS (EPVS) segmentation remains challenging due to their small size, variable morphology, similarity with other pathological features, and limited annotated datasets. This paper presents the EPVS Challenge organized at MICCAI 2024, which aims to advance the development of automated algorithms for EPVS segmentation across multi-site data. We provided a diverse dataset comprising 100 training, 50 validation, and 50 testing scans collected from multiple international sites (UK, Singapore, and China) with varying MRI protocols and demographics. All annotations followed the STRIVE protocol to ensure standardized ground truth and covered the full brain parenchyma. Seven teams completed the full challenge, implementing various deep learning approaches primarily based on U-Net architectures with innovations in multi-modal processing, ensemble strategies, and transformer-based components. Performance was evaluated using dice similarity coefficient, absolute volume difference, recall, and precision metrics. The winning method employed MedNeXt architecture with a dual 2D/3D strategy for handling varying slice thicknesses. The top solutions showed relatively good performance on test data from seen datasets, but significant degradation of performance was observed on the previously unseen Shanghai cohort, highlighting cross-site generalization challenges due to domain shift. This challenge establishes an important benchmark for EPVS segmentation methods and underscores the need for the continued development of robust algorithms that can generalize in diverse clinical settings.
Federated Learning Enables Big Data for Rare Cancer Boundary Detection
Apr 25, 2022Abstract:Although machine learning (ML) has shown promise in numerous domains, there are concerns about generalizability to out-of-sample data. This is currently addressed by centrally sharing ample, and importantly diverse, data from multiple sites. However, such centralization is challenging to scale (or even not feasible) due to various limitations. Federated ML (FL) provides an alternative to train accurate and generalizable ML models, by only sharing numerical model updates. Here we present findings from the largest FL study to-date, involving data from 71 healthcare institutions across 6 continents, to generate an automatic tumor boundary detector for the rare disease of glioblastoma, utilizing the largest dataset of such patients ever used in the literature (25,256 MRI scans from 6,314 patients). We demonstrate a 33% improvement over a publicly trained model to delineate the surgically targetable tumor, and 23% improvement over the tumor's entire extent. We anticipate our study to: 1) enable more studies in healthcare informed by large and diverse data, ensuring meaningful results for rare diseases and underrepresented populations, 2) facilitate further quantitative analyses for glioblastoma via performance optimization of our consensus model for eventual public release, and 3) demonstrate the effectiveness of FL at such scale and task complexity as a paradigm shift for multi-site collaborations, alleviating the need for data sharing.
SUNet: a deep learning architecture for acute stroke lesion segmentation and outcome prediction in multimodal MRI
Oct 31, 2018



Abstract:Acute stroke lesion segmentation and prediction tasks are of great clinical interest as they can help doctors make better informed time-critical treatment decisions. Automatic segmentation of these lesions is a complex task due to their heterogeneous appearance, dynamic evolution and inter-patient differences. Typically, acute stroke lesion tasks are approached with methods developed for chronic stroke or other brain lesions. However, the pathophysiology and anatomy of acute stroke establishes an inherently different problem that needs special consideration. In this work, we propose a novel deep learning architecture specially designed for acute stroke tasks that involve approximating complex non-linear functions with reduced data. Within our strategy, class imbalance is tackled using a hybrid strategy based on state-of-the-art train sampling strategies designed for other brain lesion related tasks, which is more suited to the anatomy and pathophysiology of acute stroke lesions. The proposed method is evaluated on three unrelated public international challenge datasets (ISLES) without any dataset specific hyper-parameter tuning. These involve the tasks of sub-acute stroke lesion segmentation, acute stroke penumbra estimation and chronic extent prediction from acute MR images. The performance of the proposed architecture is analysed both against similar deep learning architectures from chronic stroke and related biomedical tasks and also by submitting the segmented test images for blind online evaluation on each of the challenges. When compared with the rest of submitted strategies, our method achieves top-rank performance among the best submitted entries in all the three challenges, showing its capability to deal with different unrelated tasks without hyper-parameter tuning. In order to promote the reproducibility of our results, a public version of the proposed method has been released.
Deep convolutional neural networks for brain image analysis on magnetic resonance imaging: a review
Jun 11, 2018



Abstract:In recent years, deep convolutional neural networks (CNNs) have shown record-shattering performance in a variety of computer vision problems, such as visual object recognition, detection and segmentation. These methods have also been utilised in medical image analysis domain for lesion segmentation, anatomical segmentation and classification. We present an extensive literature review of CNN techniques applied in brain magnetic resonance imaging (MRI) analysis, focusing on the architectures, pre-processing, data-preparation and post-processing strategies available in these works. The aim of this study is three-fold. Our primary goal is to report how different CNN architectures have evolved, discuss state-of-the-art strategies, condense their results obtained using public datasets and examine their pros and cons. Second, this paper is intended to be a detailed reference of the research activity in deep CNN for brain MRI analysis. Finally, we present a perspective on the future of CNNs in which we hint some of the research directions in subsequent years.
Quantitative analysis of patch-based fully convolutional neural networks for tissue segmentation on brain magnetic resonance imaging
Feb 19, 2018



Abstract:Accurate brain tissue segmentation in Magnetic Resonance Imaging (MRI) has attracted the attention of medical doctors and researchers since variations in tissue volume help in diagnosing and monitoring neurological diseases. Several proposals have been designed throughout the years comprising conventional machine learning strategies as well as convolutional neural networks (CNN) approaches. In particular, in this paper, we analyse a sub-group of deep learning methods producing dense predictions. This branch, referred in the literature as Fully CNN (FCNN), is of interest as these architectures can process an input volume in less time than CNNs and local spatial dependencies may be encoded since several voxels are classified at once. Our study focuses on understanding architectural strengths and weaknesses of literature-like approaches. Hence, we implement eight FCNN architectures inspired by robust state-of-the-art methods on brain segmentation related tasks. We evaluate them using the IBSR18, MICCAI2012 and iSeg2017 datasets as they contain infant and adult data and exhibit varied voxel spacing, image quality, number of scans and available imaging modalities. The discussion is driven in three directions: comparison between 2D and 3D approaches, the importance of multiple modalities and overlapping as a sampling strategy for training and testing models. To encourage other researchers to explore the evaluation framework, a public version is accessible to download from our research website.
Automated sub-cortical brain structure segmentation combining spatial and deep convolutional features
Sep 26, 2017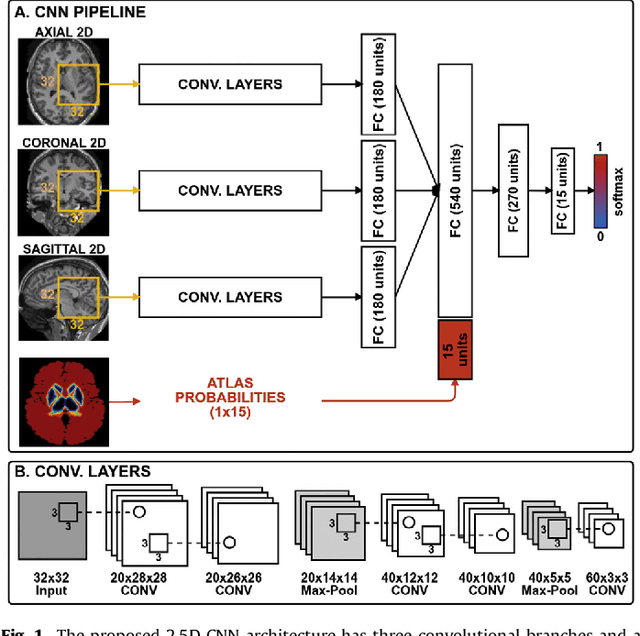
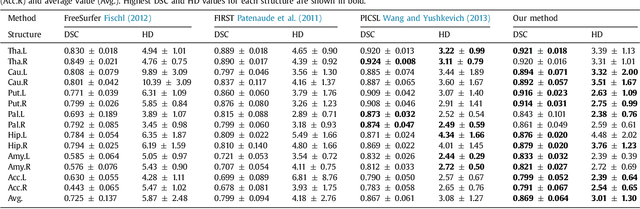
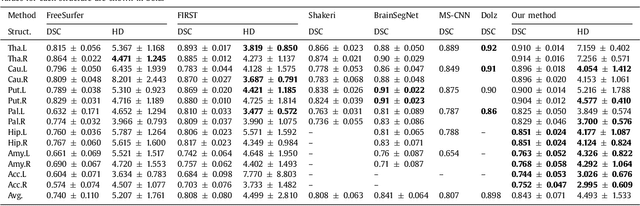
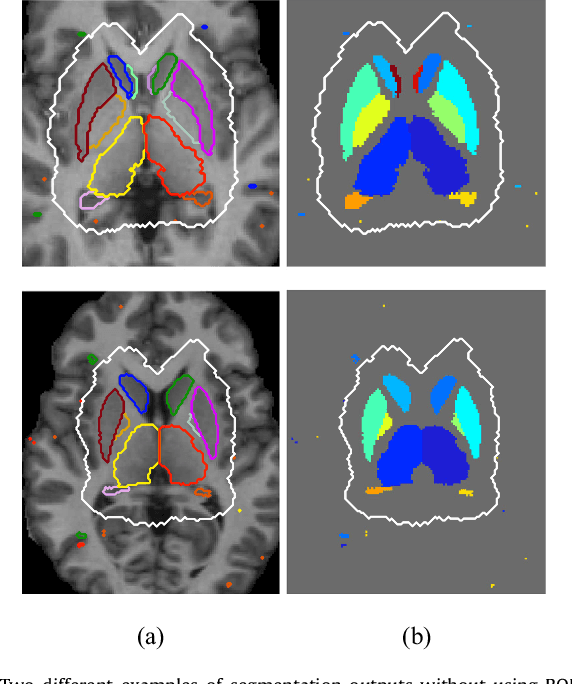
Abstract:Sub-cortical brain structure segmentation in Magnetic Resonance Images (MRI) has attracted the interest of the research community for a long time because morphological changes in these structures are related to different neurodegenerative disorders. However, manual segmentation of these structures can be tedious and prone to variability, highlighting the need for robust automated segmentation methods. In this paper, we present a novel convolutional neural network based approach for accurate segmentation of the sub-cortical brain structures that combines both convolutional and prior spatial features for improving the segmentation accuracy. In order to increase the accuracy of the automated segmentation, we propose to train the network using a restricted sample selection to force the network to learn the most difficult parts of the structures. We evaluate the accuracy of the proposed method on the public MICCAI 2012 challenge and IBSR 18 datasets, comparing it with different available state-of-the-art methods and other recently proposed deep learning approaches. On the MICCAI 2012 dataset, our method shows an excellent performance comparable to the best challenge participant strategy, while performing significantly better than state-of-the-art techniques such as FreeSurfer and FIRST. On the IBSR 18 dataset, our method also exhibits a significant increase in the performance with respect to not only FreeSurfer and FIRST, but also comparable or better results than other recent deep learning approaches. Moreover, our experiments show that both the addition of the spatial priors and the restricted sampling strategy have a significant effect on the accuracy of the proposed method. In order to encourage the reproducibility and the use of the proposed method, a public version of our approach is available to download for the neuroimaging community.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge