Jeffry Wicaksana
Non-parametric regularization for class imbalance federated medical image classification
Jul 17, 2024Abstract:Limited training data and severe class imbalance pose significant challenges to developing clinically robust deep learning models. Federated learning (FL) addresses the former by enabling different medical clients to collaboratively train a deep model without sharing privacy-sensitive data. However, class imbalance worsens due to variation in inter-client class distribution. We propose federated learning with non-parametric regularization (FedNPR and FedNPR-Per, a personalized version of FedNPR) to regularize the feature extractor and enhance useful and discriminative signal in the feature space. Our extensive experiments show that FedNPR outperform the existing state-of-the art FL approaches in class imbalance skin lesion classification and intracranial hemorrhage identification. Additionally, the non-parametric regularization module consistently improves the performance of existing state-of-the-art FL approaches. We believe that NPR is a valuable tool in FL under clinical settings.
FCA: Taming Long-tailed Federated Medical Image Classification by Classifier Anchoring
May 01, 2023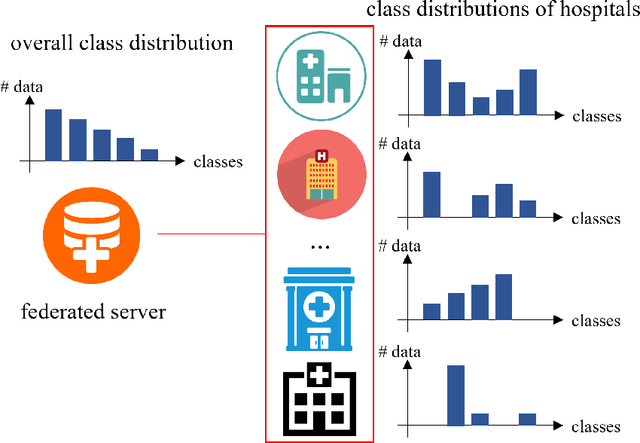
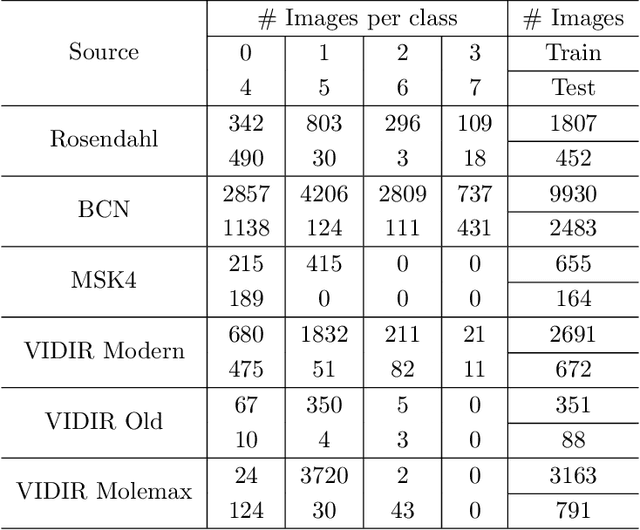
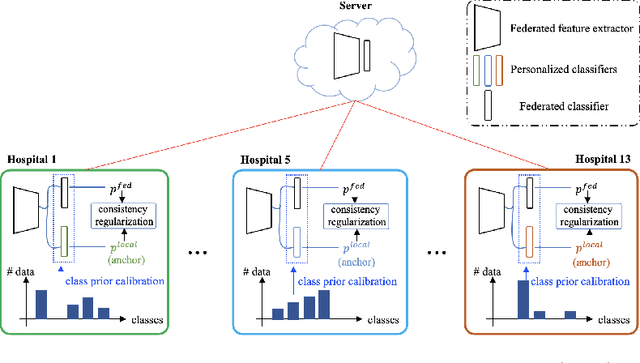
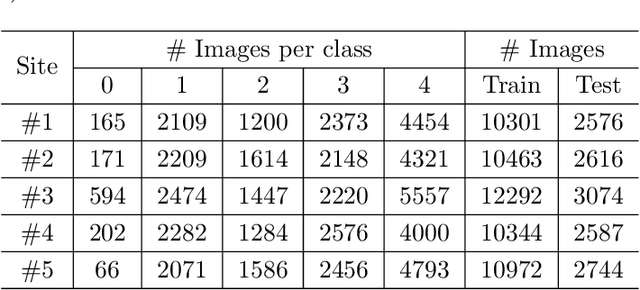
Abstract:Limited training data and severe class imbalance impose significant challenges to developing clinically robust deep learning models. Federated learning (FL) addresses the former by enabling different medical clients to collaboratively train a deep model without sharing data. However, the class imbalance problem persists due to inter-client class distribution variations. To overcome this, we propose federated classifier anchoring (FCA) by adding a personalized classifier at each client to guide and debias the federated model through consistency learning. Additionally, FCA debiases the federated classifier and each client's personalized classifier based on their respective class distributions, thus mitigating divergence. With FCA, the federated feature extractor effectively learns discriminative features suitably globally for federation as well as locally for all participants. In clinical practice, the federated model is expected to be both generalized, performing well across clients, and specialized, benefiting each individual client from collaboration. According to this, we propose a novel evaluation metric to assess models' generalization and specialization performance globally on an aggregated public test set and locally at each client. Through comprehensive comparison and evaluation, FCA outperforms the state-of-the-art methods with large margins for federated long-tailed skin lesion classification and intracranial hemorrhage classification, making it a more feasible solution in clinical settings.
SDQ: Stochastic Differentiable Quantization with Mixed Precision
Jun 17, 2022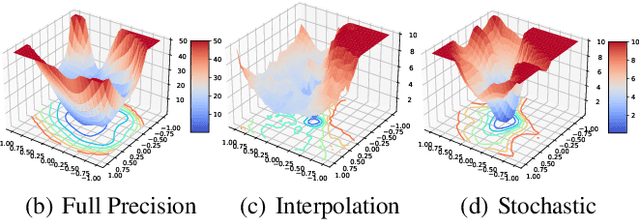
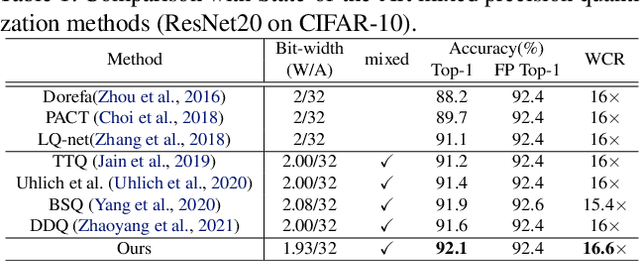
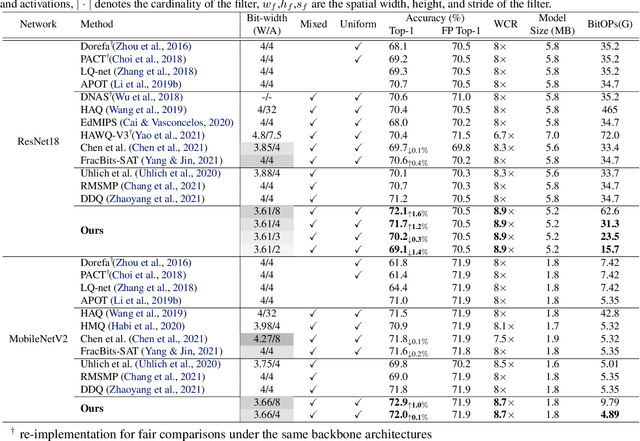
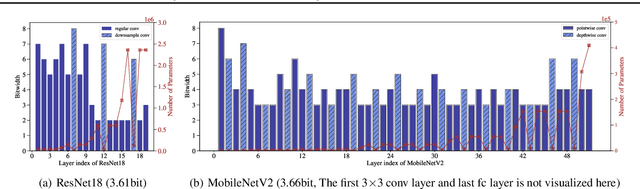
Abstract:In order to deploy deep models in a computationally efficient manner, model quantization approaches have been frequently used. In addition, as new hardware that supports mixed bitwidth arithmetic operations, recent research on mixed precision quantization (MPQ) begins to fully leverage the capacity of representation by searching optimized bitwidths for different layers and modules in a network. However, previous studies mainly search the MPQ strategy in a costly scheme using reinforcement learning, neural architecture search, etc., or simply utilize partial prior knowledge for bitwidth assignment, which might be biased and sub-optimal. In this work, we present a novel Stochastic Differentiable Quantization (SDQ) method that can automatically learn the MPQ strategy in a more flexible and globally-optimized space with smoother gradient approximation. Particularly, Differentiable Bitwidth Parameters (DBPs) are employed as the probability factors in stochastic quantization between adjacent bitwidth choices. After the optimal MPQ strategy is acquired, we further train our network with entropy-aware bin regularization and knowledge distillation. We extensively evaluate our method for several networks on different hardware (GPUs and FPGA) and datasets. SDQ outperforms all state-of-the-art mixed or single precision quantization with a lower bitwidth and is even better than the full-precision counterparts across various ResNet and MobileNet families, demonstrating the effectiveness and superiority of our method.
FedMix: Mixed Supervised Federated Learning for Medical Image Segmentation
May 04, 2022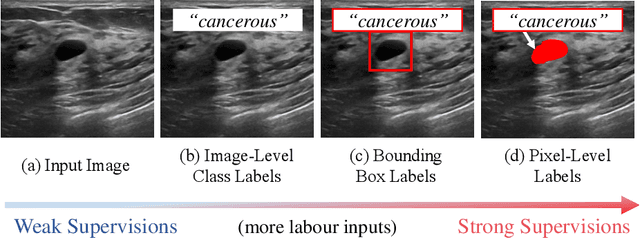
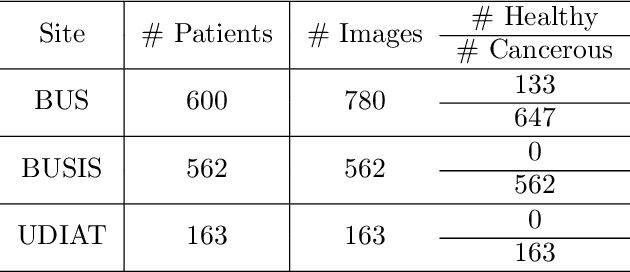
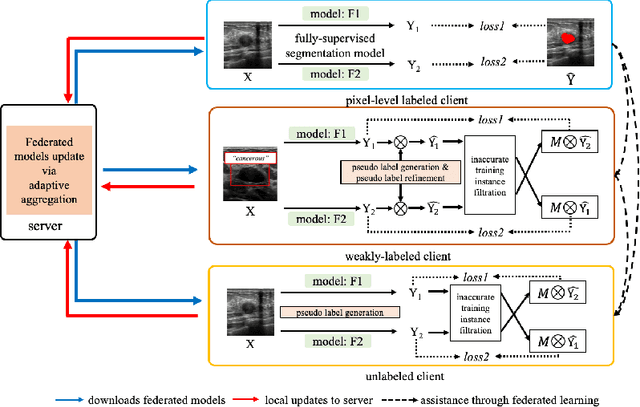
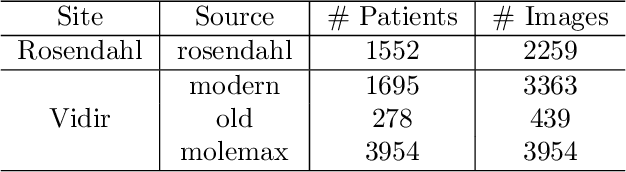
Abstract:The purpose of federated learning is to enable multiple clients to jointly train a machine learning model without sharing data. However, the existing methods for training an image segmentation model have been based on an unrealistic assumption that the training set for each local client is annotated in a similar fashion and thus follows the same image supervision level. To relax this assumption, in this work, we propose a label-agnostic unified federated learning framework, named FedMix, for medical image segmentation based on mixed image labels. In FedMix, each client updates the federated model by integrating and effectively making use of all available labeled data ranging from strong pixel-level labels, weak bounding box labels, to weakest image-level class labels. Based on these local models, we further propose an adaptive weight assignment procedure across local clients, where each client learns an aggregation weight during the global model update. Compared to the existing methods, FedMix not only breaks through the constraint of a single level of image supervision, but also can dynamically adjust the aggregation weight of each local client, achieving rich yet discriminative feature representations. To evaluate its effectiveness, experiments have been carried out on two challenging medical image segmentation tasks, i.e., breast tumor segmentation and skin lesion segmentation. The results validate that our proposed FedMix outperforms the state-of-the-art method by a large margin.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge