Huihui Huang
SecureAgentBench: Benchmarking Secure Code Generation under Realistic Vulnerability Scenarios
Sep 26, 2025Abstract:Large language model (LLM) powered code agents are rapidly transforming software engineering by automating tasks such as testing, debugging, and repairing, yet the security risks of their generated code have become a critical concern. Existing benchmarks have offered valuable insights but remain insufficient: they often overlook the genuine context in which vulnerabilities were introduced or adopt narrow evaluation protocols that fail to capture either functional correctness or newly introduced vulnerabilities. We therefore introduce SecureAgentBench, a benchmark of 105 coding tasks designed to rigorously evaluate code agents' capabilities in secure code generation. Each task includes (i) realistic task settings that require multi-file edits in large repositories, (ii) aligned contexts based on real-world open-source vulnerabilities with precisely identified introduction points, and (iii) comprehensive evaluation that combines functionality testing, vulnerability checking through proof-of-concept exploits, and detection of newly introduced vulnerabilities using static analysis. We evaluate three representative agents (SWE-agent, OpenHands, and Aider) with three state-of-the-art LLMs (Claude 3.7 Sonnet, GPT-4.1, and DeepSeek-V3.1). Results show that (i) current agents struggle to produce secure code, as even the best-performing one, SWE-agent supported by DeepSeek-V3.1, achieves merely 15.2% correct-and-secure solutions, (ii) some agents produce functionally correct code but still introduce vulnerabilities, including new ones not previously recorded, and (iii) adding explicit security instructions for agents does not significantly improve secure coding, underscoring the need for further research. These findings establish SecureAgentBench as a rigorous benchmark for secure code generation and a step toward more reliable software development with LLMs.
R2Vul: Learning to Reason about Software Vulnerabilities with Reinforcement Learning and Structured Reasoning Distillation
Apr 07, 2025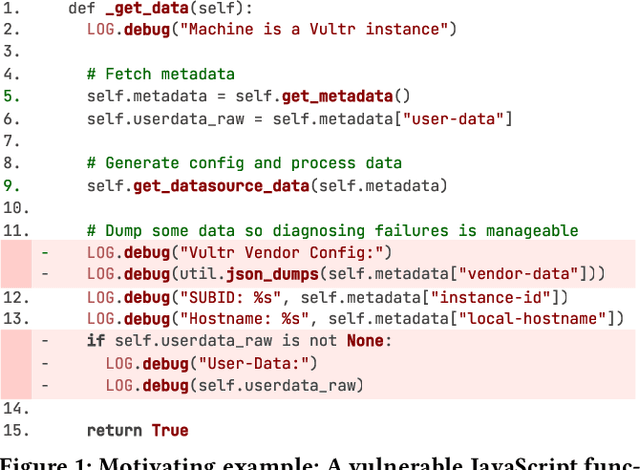


Abstract:Large language models (LLMs) have shown promising performance in software vulnerability detection (SVD), yet their reasoning capabilities remain unreliable. Existing approaches relying on chain-of-thought (CoT) struggle to provide relevant and actionable security assessments. Additionally, effective SVD requires not only generating coherent reasoning but also differentiating between well-founded and misleading yet plausible security assessments, an aspect overlooked in prior work. To this end, we introduce R2Vul, a novel approach that distills structured reasoning into small LLMs using reinforcement learning from AI feedback (RLAIF). Through RLAIF, R2Vul enables LLMs to produce structured, security-aware reasoning that is actionable and reliable while explicitly learning to distinguish valid assessments from misleading ones. We evaluate R2Vul across five languages against SAST tools, CoT, instruction tuning, and classification-based baselines. Our results show that R2Vul with structured reasoning distillation enables a 1.5B student LLM to rival larger models while improving generalization to out-of-distribution vulnerabilities. Beyond model improvements, we contribute a large-scale, multilingual preference dataset featuring structured reasoning to support future research in SVD.
A Cross Spatio-Temporal Pathology-based Lung Nodule Dataset
Jun 26, 2024Abstract:Recently, intelligent analysis of lung nodules with the assistant of computer aided detection (CAD) techniques can improve the accuracy rate of lung cancer diagnosis. However, existing CAD systems and pulmonary datasets mainly focus on Computed Tomography (CT) images from one single period, while ignoring the cross spatio-temporal features associated with the progression of nodules contained in imaging data from various captured periods of lung cancer. If the evolution patterns of nodules across various periods in the patients' CT sequences can be explored, it will play a crucial role in guiding the precise screening identification of lung cancer. Therefore, a cross spatio-temporal lung nodule dataset with pathological information for nodule identification and diagnosis is constructed, which contains 328 CT sequences and 362 annotated nodules from 109 patients. This comprehensive database is intended to drive research in the field of CAD towards more practical and robust methods, and also contribute to the further exploration of precision medicine related field. To ensure patient confidentiality, we have removed sensitive information from the dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge