Horst K. Hahn
Requirements for Quality Assurance of AI Models for Early Detection of Lung Cancer
Feb 24, 2025Abstract:Lung cancer is the second most common cancer and the leading cause of cancer-related deaths worldwide. Survival largely depends on tumor stage at diagnosis, and early detection with low-dose CT can significantly reduce mortality in high-risk patients. AI can improve the detection, measurement, and characterization of pulmonary nodules while reducing assessment time. However, the training data, functionality, and performance of available AI systems vary considerably, complicating software selection and regulatory evaluation. Manufacturers must specify intended use and provide test statistics, but they can choose their training and test data, limiting standardization and comparability. Under the EU AI Act, consistent quality assurance is required for AI-based nodule detection, measurement, and characterization. This position paper proposes systematic quality assurance grounded in a validated reference dataset, including real screening cases plus phantom data to verify volume and growth rate measurements. Regular updates shall reflect demographic shifts and technological advances, ensuring ongoing relevance. Consequently, ongoing AI quality assurance is vital. Regulatory challenges are also adressed. While the MDR and the EU AI Act set baseline requirements, they do not adequately address self-learning algorithms or their updates. A standardized, transparent quality assessment - based on sensitivity, specificity, and volumetric accuracy - enables an objective evaluation of each AI solution's strengths and weaknesses. Establishing clear testing criteria and systematically using updated reference data lay the groundwork for comparable performance metrics, informing tenders, guidelines, and recommendations.
Robust Segmentation Models using an Uncertainty Slice Sampling Based Annotation Workflow
Sep 30, 2021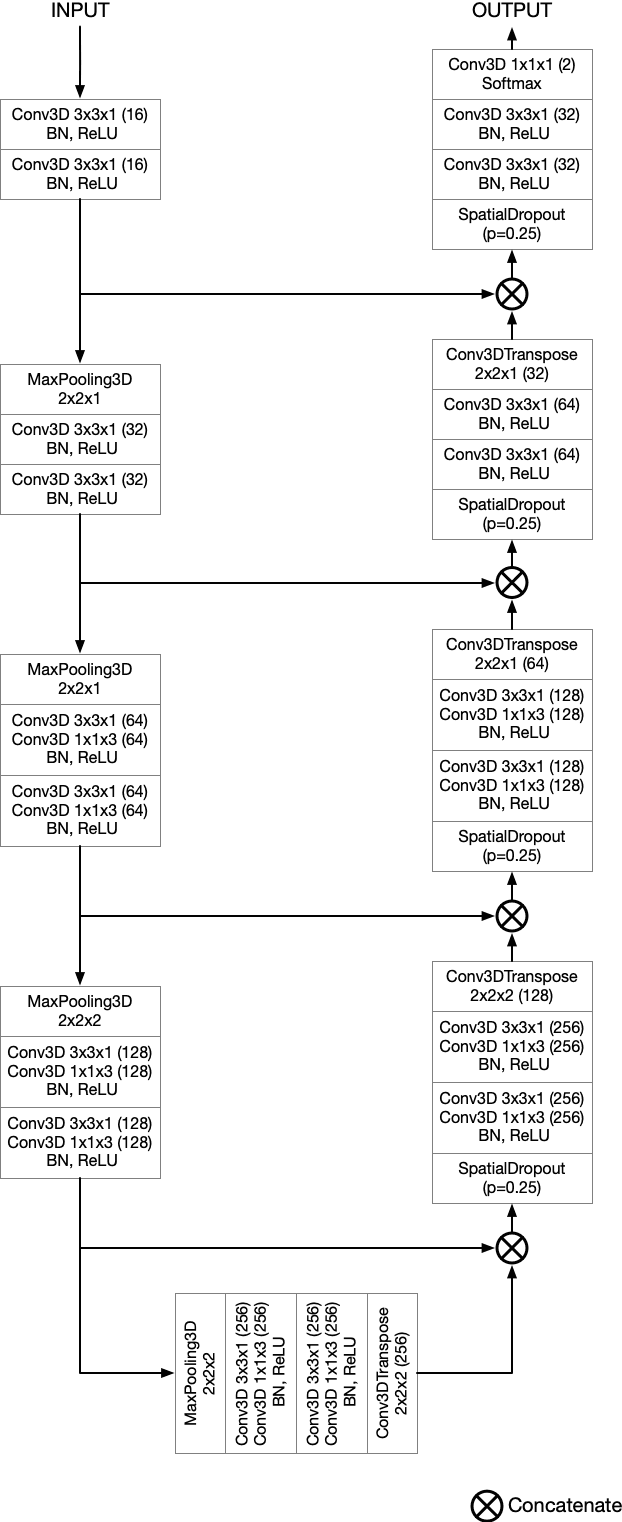
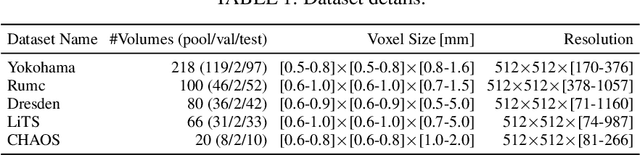
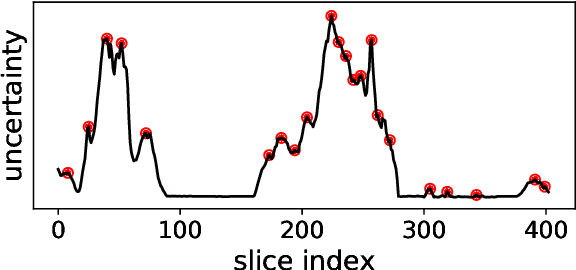
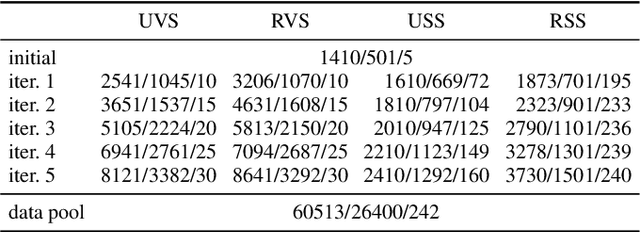
Abstract:Semantic segmentation neural networks require pixel-level annotations in large quantities to achieve a good performance. In the medical domain, such annotations are expensive, because they are time-consuming and require expert knowledge. Active learning optimizes the annotation effort by devising strategies to select cases for labeling that are most informative to the model. In this work, we propose an uncertainty slice sampling (USS) strategy for semantic segmentation of 3D medical volumes that selects 2D image slices for annotation and compare it with various other strategies. We demonstrate the efficiency of USS on a CT liver segmentation task using multi-site data. After five iterations, the training data resulting from USS consisted of 2410 slices (4% of all slices in the data pool) compared to 8121 (13%), 8641 (14%), and 3730 (6%) for uncertainty volume (UVS), random volume (RVS), and random slice (RSS) sampling, respectively. Despite being trained on the smallest amount of data, the model based on the USS strategy evaluated on 234 test volumes significantly outperformed models trained according to other strategies and achieved a mean Dice index of 0.964, a relative volume error of 4.2%, a mean surface distance of 1.35 mm, and a Hausdorff distance of 23.4 mm. This was only slightly inferior to 0.967, 3.8%, 1.18 mm, and 22.9 mm achieved by a model trained on all available data, but the robustness analysis using the 5th percentile of Dice and the 95th percentile of the remaining metrics demonstrated that USS resulted not only in the most robust model compared to other sampling schemes, but also outperformed the model trained on all data according to Dice (0.946 vs. 0.945) and mean surface distance (1.92 mm vs. 2.03 mm).
Anisotropic 3D Multi-Stream CNN for Accurate Prostate Segmentation from Multi-Planar MRI
Sep 23, 2020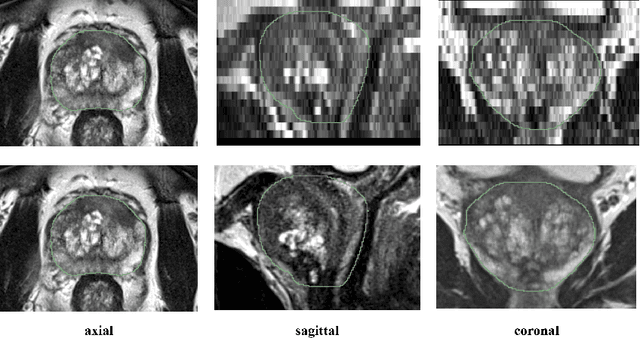
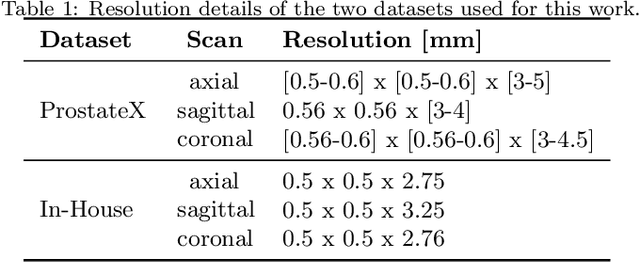
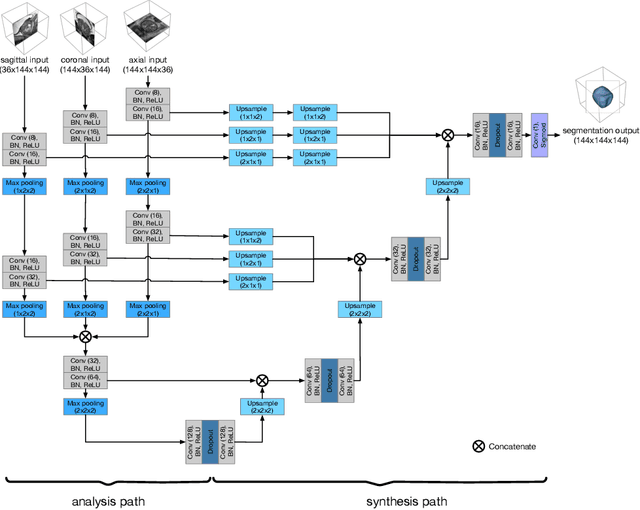
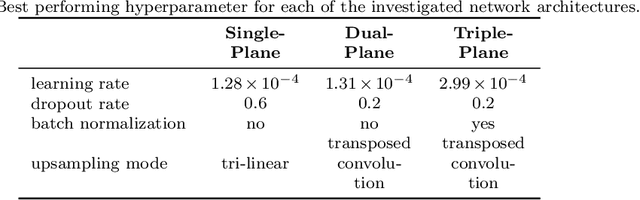
Abstract:Background and Objective: Accurate and reliable segmentation of the prostate gland in MR images can support the clinical assessment of prostate cancer, as well as the planning and monitoring of focal and loco-regional therapeutic interventions. Despite the availability of multi-planar MR scans due to standardized protocols, the majority of segmentation approaches presented in the literature consider the axial scans only. Methods: We propose an anisotropic 3D multi-stream CNN architecture, which processes additional scan directions to produce a higher-resolution isotropic prostate segmentation. We investigate two variants of our architecture, which work on two (dual-plane) and three (triple-plane) image orientations, respectively. We compare them with the standard baseline (single-plane) used in literature, i.e., plain axial segmentation. To realize a fair comparison, we employ a hyperparameter optimization strategy to select optimal configurations for the individual approaches. Results: Training and evaluation on two datasets spanning multiple sites obtain statistical significant improvement over the plain axial segmentation ($p<0.05$ on the Dice similarity coefficient). The improvement can be observed especially at the base ($0.898$ single-plane vs. $0.906$ triple-plane) and apex ($0.888$ single-plane vs. $0.901$ dual-plane). Conclusion: This study indicates that models employing two or three scan directions are superior to plain axial segmentation. The knowledge of precise boundaries of the prostate is crucial for the conservation of risk structures. Thus, the proposed models have the potential to improve the outcome of prostate cancer diagnosis and therapies.
A Ray-based Approach for Boundary Estimation of Fiber Bundles Derived from Diffusion Tensor Imaging
Oct 23, 2013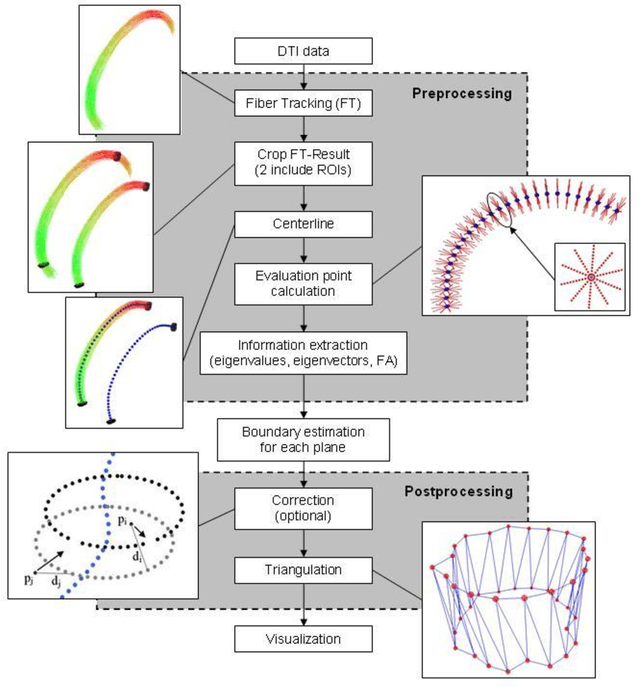
Abstract:Diffusion Tensor Imaging (DTI) is a non-invasive imaging technique that allows estimation of the location of white matter tracts in-vivo, based on the measurement of water diffusion properties. For each voxel, a second-order tensor can be calculated by using diffusion-weighted sequences (DWI) that are sensitive to the random motion of water molecules. Given at least 6 diffusion-weighted images with different gradients and one unweighted image, the coefficients of the symmetric diffusion tensor matrix can be calculated. Deriving the eigensystem of the tensor, the eigenvectors and eigenvalues can be calculated to describe the three main directions of diffusion and its magnitude. Using DTI data, fiber bundles can be determined, to gain information about eloquent brain structures. Especially in neurosurgery, information about location and dimension of eloquent structures like the corticospinal tract or the visual pathways is of major interest. Therefore, the fiber bundle boundary has to be determined. In this paper, a novel ray-based approach for boundary estimation of tubular structures is presented.
* 5 pages, 2 figures, 7 references
Benchmarking the Quality of Diffusion-Weighted Images
May 09, 2011
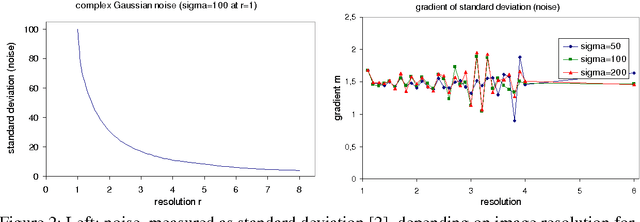
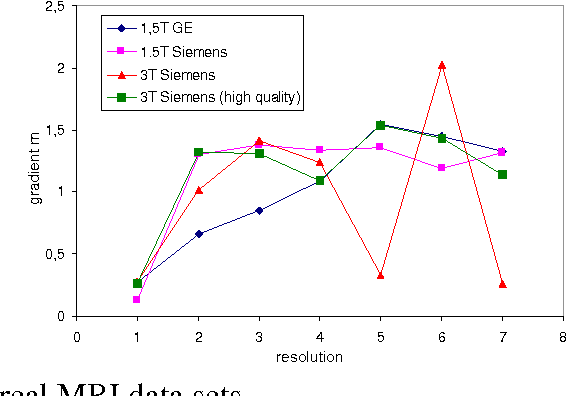
Abstract:We present a novel method that allows for measuring the quality of diffusion-weighted MR images dependent on the image resolution and the image noise. For this purpose, we introduce a new thresholding technique so that noise and the signal can automatically be estimated from a single data set. Thus, no user interaction as well as no double acquisition technique, which requires a time-consuming proper geometrical registration, is needed. As a coarser image resolution or slice thickness leads to a higher signal-to-noise ratio (SNR), our benchmark determines a resolution-independent quality measure so that images with different resolutions can be adequately compared. To evaluate our method, a set of diffusion-weighted images from different vendors is used. It is shown that the quality can efficiently be determined and that the automatically computed SNR is comparable to the SNR which is measured manually in a manually selected region of interest.
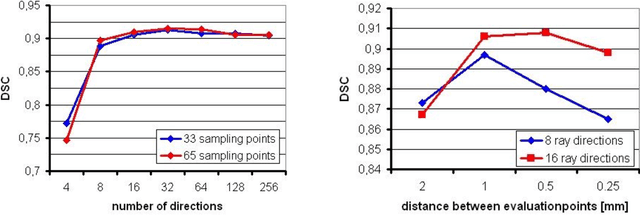
Ray-Based and Graph-Based Methods for Fiber Bundle Boundary Estimation
Mar 10, 2011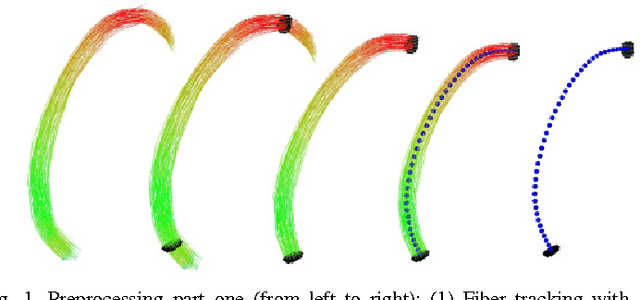
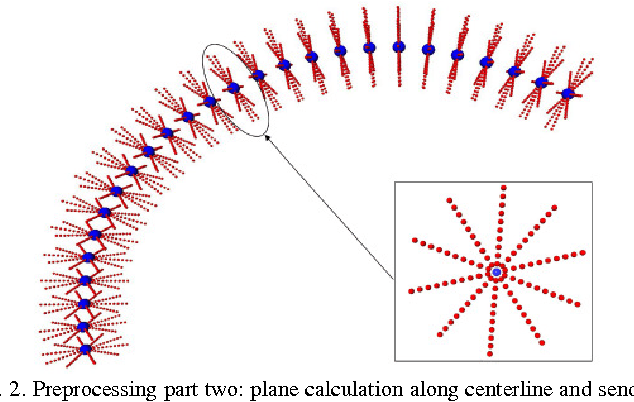
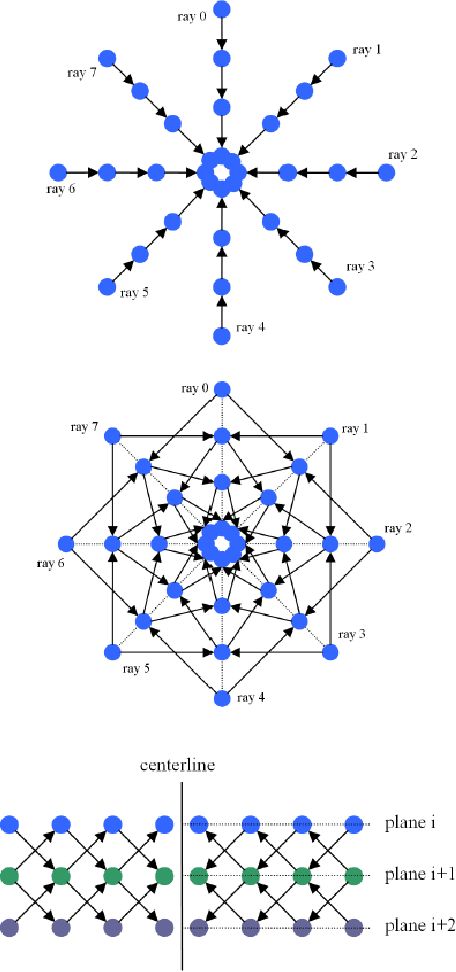
Abstract:Diffusion Tensor Imaging (DTI) provides the possibility of estimating the location and course of eloquent structures in the human brain. Knowledge about this is of high importance for preoperative planning of neurosurgical interventions and for intraoperative guidance by neuronavigation in order to minimize postoperative neurological deficits. Therefore, the segmentation of these structures as closed, three-dimensional object is necessary. In this contribution, two methods for fiber bundle segmentation between two defined regions are compared using software phantoms (abstract model and anatomical phantom modeling the right corticospinal tract). One method uses evaluation points from sampled rays as candidates for boundary points, the other method sets up a directed and weighted (depending on a scalar measure) graph and performs a min-cut for optimal segmentation results. Comparison is done by using the Dice Similarity Coefficient (DSC), a measure for spatial overlap of different segmentation results.
A Semi-Automatic Graph-Based Approach for Determining the Boundary of Eloquent Fiber Bundles in the Human Brain
Mar 08, 2011Abstract:Diffusion Tensor Imaging (DTI) allows estimating the position, orientation and dimension of bundles of nerve pathways. This non-invasive imaging technique takes advantage of the diffusion of water molecules and determines the diffusion coefficients for every voxel of the data set. The identification of the diffusion coefficients and the derivation of information about fiber bundles is of major interest for planning and performing neurosurgical interventions. To minimize the risk of neural deficits during brain surgery as tumor resection (e.g. glioma), the segmentation and integration of the results in the operating room is of prime importance. In this contribution, a robust and efficient graph-based approach for segmentating tubular fiber bundles in the human brain is presented. To define a cost function, the fractional anisotropy (FA) is used, derived from the DTI data, but this value may differ from patient to patient. Besides manually definining seed regions describing the structure of interest, additionally a manual definition of the cost function by the user is necessary. To improve the approach the contribution introduces a solution for automatically determining the cost function by using different 3D masks for each individual data set.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge