Hongbo Chu
Agent Aggregator with Mask Denoise Mechanism for Histopathology Whole Slide Image Analysis
Sep 18, 2024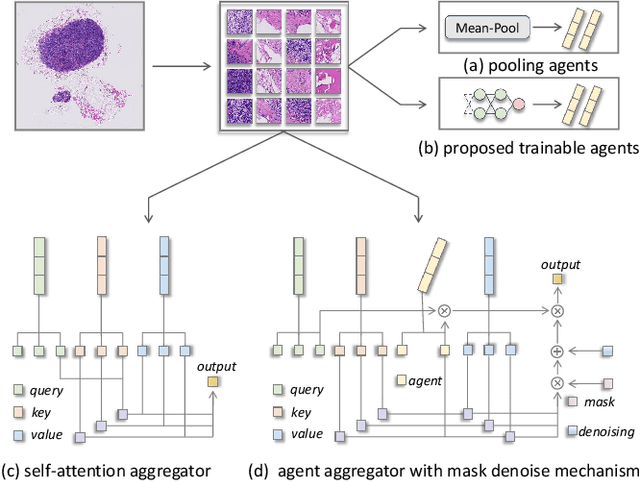
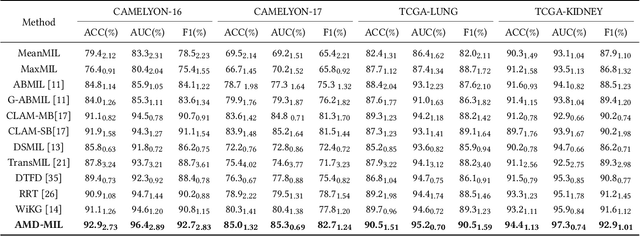
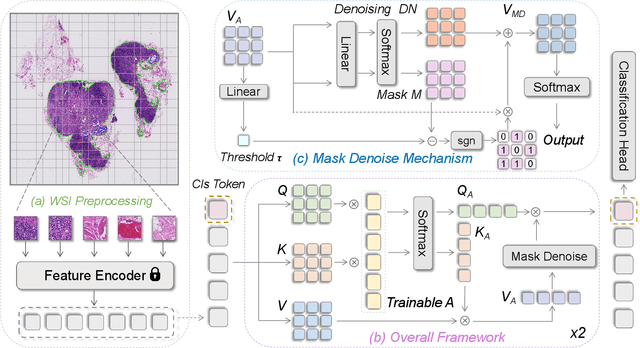
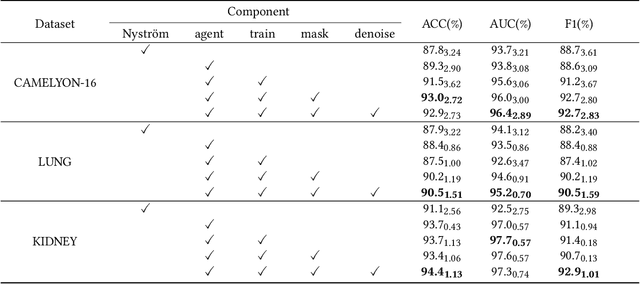
Abstract:Histopathology analysis is the gold standard for medical diagnosis. Accurate classification of whole slide images (WSIs) and region-of-interests (ROIs) localization can assist pathologists in diagnosis. The gigapixel resolution of WSI and the absence of fine-grained annotations make direct classification and analysis challenging. In weakly supervised learning, multiple instance learning (MIL) presents a promising approach for WSI classification. The prevailing strategy is to use attention mechanisms to measure instance importance for classification. However, attention mechanisms fail to capture inter-instance information, and self-attention causes quadratic computational complexity. To address these challenges, we propose AMD-MIL, an agent aggregator with a mask denoise mechanism. The agent token acts as an intermediate variable between the query and key for computing instance importance. Mask and denoising matrices, mapped from agents-aggregated value, dynamically mask low-contribution representations and eliminate noise. AMD-MIL achieves better attention allocation by adjusting feature representations, capturing micro-metastases in cancer, and improving interpretability. Extensive experiments on CAMELYON-16, CAMELYON-17, TCGA-KIDNEY, and TCGA-LUNG show AMD-MIL's superiority over state-of-the-art methods.
RetMIL: Retentive Multiple Instance Learning for Histopathological Whole Slide Image Classification
Mar 16, 2024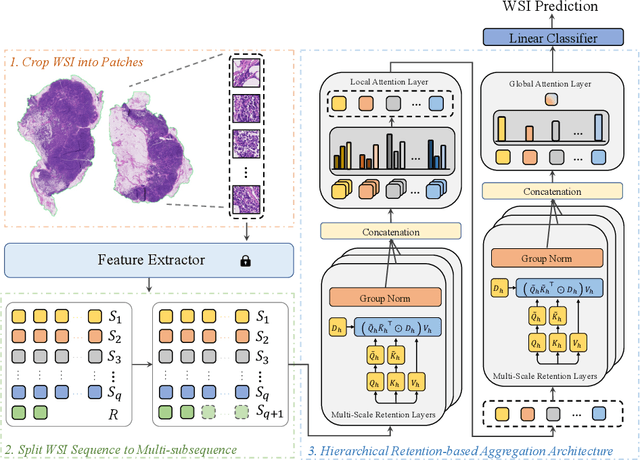
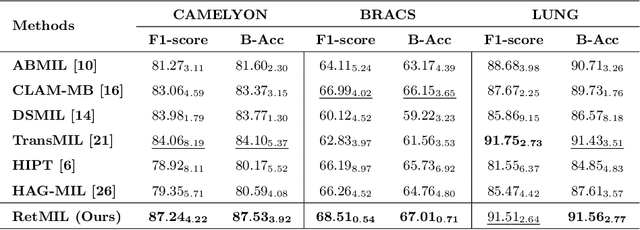
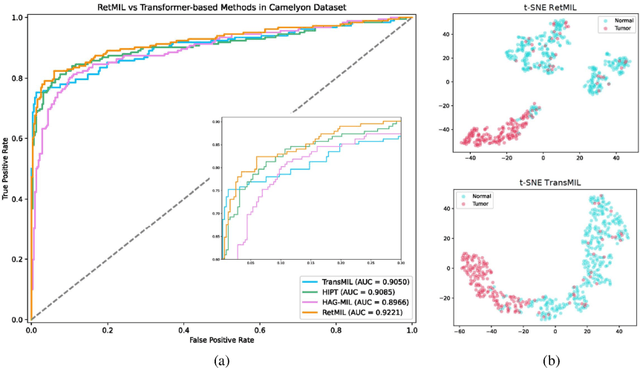
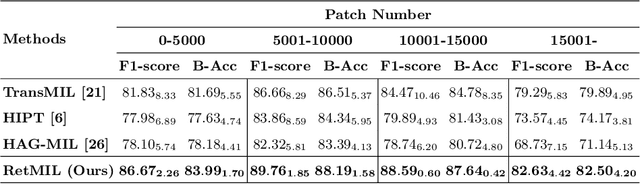
Abstract:Histopathological whole slide image (WSI) analysis with deep learning has become a research focus in computational pathology. The current paradigm is mainly based on multiple instance learning (MIL), in which approaches with Transformer as the backbone are well discussed. These methods convert WSI tasks into sequence tasks by representing patches as tokens in the WSI sequence. However, the feature complexity brought by high heterogeneity and the ultra-long sequences brought by gigapixel size makes Transformer-based MIL suffer from the challenges of high memory consumption, slow inference speed, and lack of performance. To this end, we propose a retentive MIL method called RetMIL, which processes WSI sequences through hierarchical feature propagation structure. At the local level, the WSI sequence is divided into multiple subsequences. Tokens of each subsequence are updated through a parallel linear retention mechanism and aggregated utilizing an attention layer. At the global level, subsequences are fused into a global sequence, then updated through a serial retention mechanism, and finally the slide-level representation is obtained through a global attention pooling. We conduct experiments on two public CAMELYON and BRACS datasets and an public-internal LUNG dataset, confirming that RetMIL not only achieves state-of-the-art performance but also significantly reduces computational overhead. Our code will be accessed shortly.
Dynamic Graph Representation with Knowledge-aware Attention for Histopathology Whole Slide Image Analysis
Mar 12, 2024



Abstract:Histopathological whole slide images (WSIs) classification has become a foundation task in medical microscopic imaging processing. Prevailing approaches involve learning WSIs as instance-bag representations, emphasizing significant instances but struggling to capture the interactions between instances. Additionally, conventional graph representation methods utilize explicit spatial positions to construct topological structures but restrict the flexible interaction capabilities between instances at arbitrary locations, particularly when spatially distant. In response, we propose a novel dynamic graph representation algorithm that conceptualizes WSIs as a form of the knowledge graph structure. Specifically, we dynamically construct neighbors and directed edge embeddings based on the head and tail relationships between instances. Then, we devise a knowledge-aware attention mechanism that can update the head node features by learning the joint attention score of each neighbor and edge. Finally, we obtain a graph-level embedding through the global pooling process of the updated head, serving as an implicit representation for the WSI classification. Our end-to-end graph representation learning approach has outperformed the state-of-the-art WSI analysis methods on three TCGA benchmark datasets and in-house test sets. Our code is available at https://github.com/WonderLandxD/WiKG.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge