Gyutaek Oh
AutoMedic: An Automated Evaluation Framework for Clinical Conversational Agents with Medical Dataset Grounding
Dec 11, 2025Abstract:Evaluating large language models (LLMs) has recently emerged as a critical issue for safe and trustworthy application of LLMs in the medical domain. Although a variety of static medical question-answering (QA) benchmarks have been proposed, many aspects remain underexplored, such as the effectiveness of LLMs in generating responses in dynamic, interactive clinical multi-turn conversation situations and the identification of multi-faceted evaluation strategies beyond simple accuracy. However, formally evaluating a dynamic, interactive clinical situation is hindered by its vast combinatorial space of possible patient states and interaction trajectories, making it difficult to standardize and quantitatively measure such scenarios. Here, we introduce AutoMedic, a multi-agent simulation framework that enables automated evaluation of LLMs as clinical conversational agents. AutoMedic transforms off-the-shelf static QA datasets into virtual patient profiles, enabling realistic and clinically grounded multi-turn clinical dialogues between LLM agents. The performance of various clinical conversational agents is then assessed based on our CARE metric, which provides a multi-faceted evaluation standard of clinical conversational accuracy, efficiency/strategy, empathy, and robustness. Our findings, validated by human experts, demonstrate the validity of AutoMedic as an automated evaluation framework for clinical conversational agents, offering practical guidelines for the effective development of LLMs in conversational medical applications.
Rethinking Test-Time Scaling for Medical AI: Model and Task-Aware Strategies for LLMs and VLMs
Jun 16, 2025



Abstract:Test-time scaling has recently emerged as a promising approach for enhancing the reasoning capabilities of large language models or vision-language models during inference. Although a variety of test-time scaling strategies have been proposed, and interest in their application to the medical domain is growing, many critical aspects remain underexplored, including their effectiveness for vision-language models and the identification of optimal strategies for different settings. In this paper, we conduct a comprehensive investigation of test-time scaling in the medical domain. We evaluate its impact on both large language models and vision-language models, considering factors such as model size, inherent model characteristics, and task complexity. Finally, we assess the robustness of these strategies under user-driven factors, such as misleading information embedded in prompts. Our findings offer practical guidelines for the effective use of test-time scaling in medical applications and provide insights into how these strategies can be further refined to meet the reliability and interpretability demands of the medical domain.
scHyena: Foundation Model for Full-Length Single-Cell RNA-Seq Analysis in Brain
Oct 04, 2023Abstract:Single-cell RNA sequencing (scRNA-seq) has made significant strides in unraveling the intricate cellular diversity within complex tissues. This is particularly critical in the brain, presenting a greater diversity of cell types than other tissue types, to gain a deeper understanding of brain function within various cellular contexts. However, analyzing scRNA-seq data remains a challenge due to inherent measurement noise stemming from dropout events and the limited utilization of extensive gene expression information. In this work, we introduce scHyena, a foundation model designed to address these challenges and enhance the accuracy of scRNA-seq analysis in the brain. Specifically, inspired by the recent Hyena operator, we design a novel Transformer architecture called singe-cell Hyena (scHyena) that is equipped with a linear adaptor layer, the positional encoding via gene-embedding, and a {bidirectional} Hyena operator. This enables us to process full-length scRNA-seq data without losing any information from the raw data. In particular, our model learns generalizable features of cells and genes through pre-training scHyena using the full length of scRNA-seq data. We demonstrate the superior performance of scHyena compared to other benchmark methods in downstream tasks, including cell type classification and scRNA-seq imputation.
Unpaired Deep Learning for Pharmacokinetic Parameter Estimation from Dynamic Contrast-Enhanced MRI
Jun 07, 2023



Abstract:DCE-MRI provides information about vascular permeability and tissue perfusion through the acquisition of pharmacokinetic parameters. However, traditional methods for estimating these pharmacokinetic parameters involve fitting tracer kinetic models, which often suffer from computational complexity and low accuracy due to noisy arterial input function (AIF) measurements. Although some deep learning approaches have been proposed to tackle these challenges, most existing methods rely on supervised learning that requires paired input DCE-MRI and labeled pharmacokinetic parameter maps. This dependency on labeled data introduces significant time and resource constraints, as well as potential noise in the labels, making supervised learning methods often impractical. To address these limitations, here we present a novel unpaired deep learning method for estimating both pharmacokinetic parameters and the AIF using a physics-driven CycleGAN approach. Our proposed CycleGAN framework is designed based on the underlying physics model, resulting in a simpler architecture with a single generator and discriminator pair. Crucially, our experimental results indicate that our method, which does not necessitate separate AIF measurements, produces more reliable pharmacokinetic parameters than other techniques.
Annealed Score-Based Diffusion Model for MR Motion Artifact Reduction
Jan 08, 2023Abstract:Motion artifact reduction is one of the important research topics in MR imaging, as the motion artifact degrades image quality and makes diagnosis difficult. Recently, many deep learning approaches have been studied for motion artifact reduction. Unfortunately, most existing models are trained in a supervised manner, requiring paired motion-corrupted and motion-free images, or are based on a strict motion-corruption model, which limits their use for real-world situations. To address this issue, here we present an annealed score-based diffusion model for MRI motion artifact reduction. Specifically, we train a score-based model using only motion-free images, and then motion artifacts are removed by applying forward and reverse diffusion processes repeatedly to gradually impose a low-frequency data consistency. Experimental results verify that the proposed method successfully reduces both simulated and in vivo motion artifacts, outperforming the state-of-the-art deep learning methods.
CycleQSM: Unsupervised QSM Deep Learning using Physics-Informed CycleGAN
Dec 07, 2020
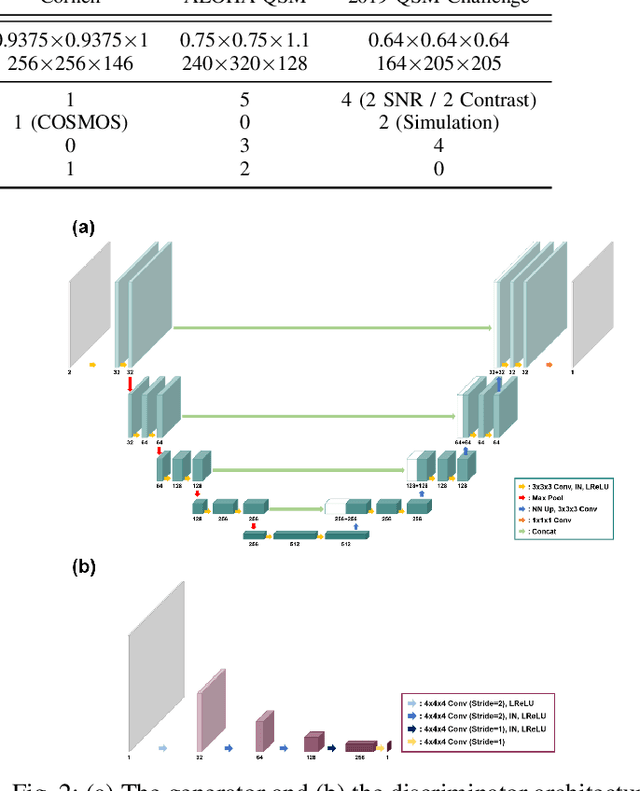
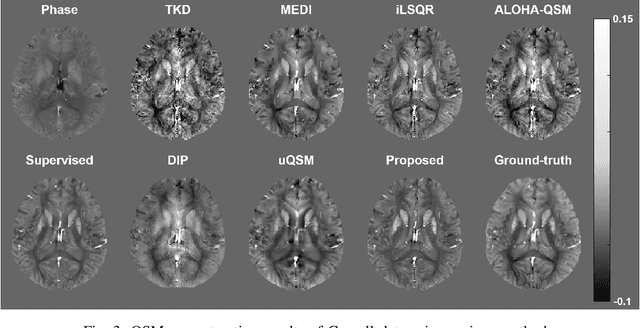
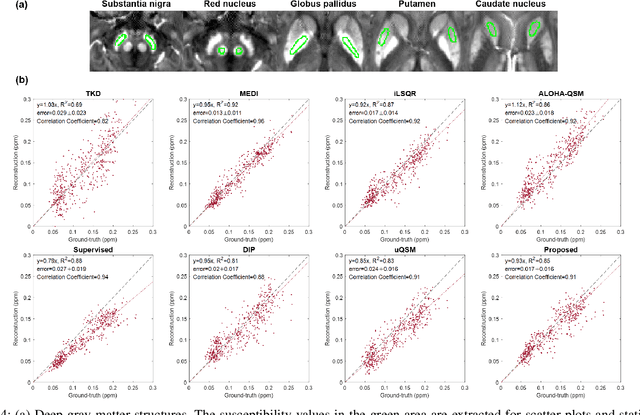
Abstract:Quantitative susceptibility mapping (QSM) is a useful magnetic resonance imaging (MRI) technique which provides spatial distribution of magnetic susceptibility values of tissues. QSMs can be obtained by deconvolving the dipole kernel from phase images, but the spectral nulls in the dipole kernel make the inversion ill-posed. In recent times, deep learning approaches have shown a comparable QSM reconstruction performance as the classic approaches, despite the fast reconstruction time. Most of the existing deep learning methods are, however, based on supervised learning, so matched pairs of input phase images and the ground-truth maps are needed. Moreover, it was reported that the supervised learning often leads to underestimated QSM values. To address this, here we propose a novel unsupervised QSM deep learning method using physics-informed cycleGAN, which is derived from optimal transport perspective. In contrast to the conventional cycleGAN, our novel cycleGAN has only one generator and one discriminator thanks to the known dipole kernel. Experimental results confirm that the proposed method provides more accurate QSM maps compared to the existing deep learning approaches, and provide competitive performance to the best classical approaches despite the ultra-fast reconstruction.
Unsupervised MR Motion Artifact Deep Learning using Outlier-Rejecting Bootstrap Aggregation
Nov 12, 2020
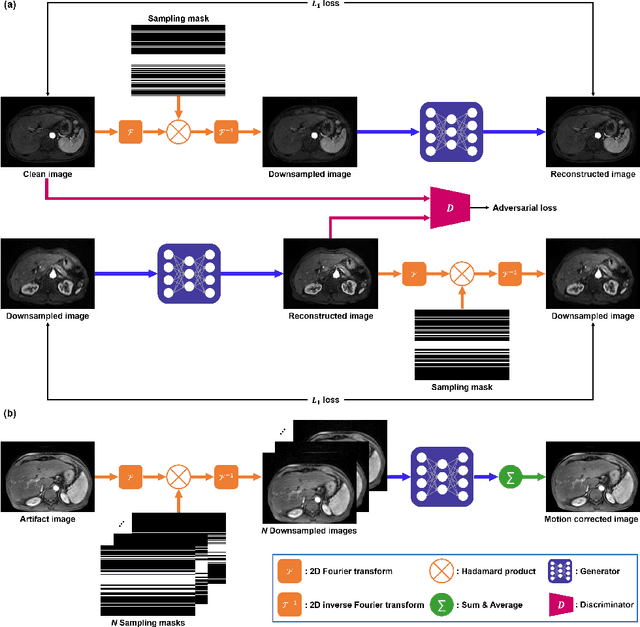
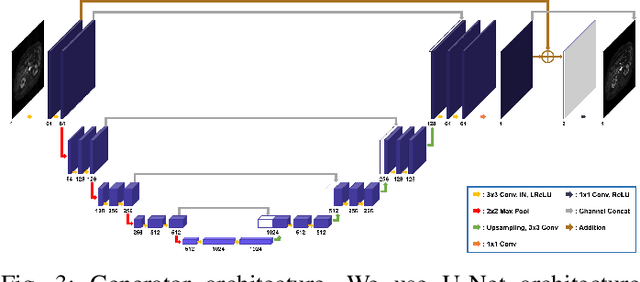
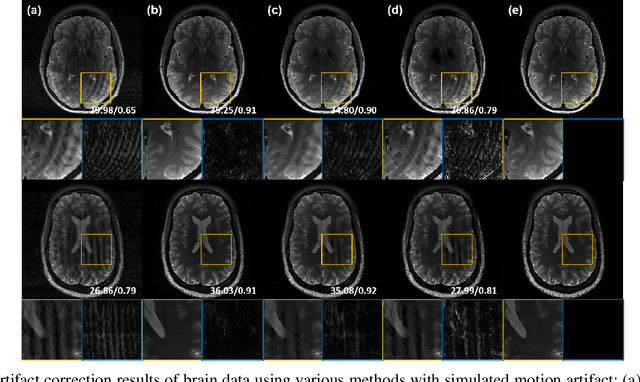
Abstract:Recently, deep learning approaches for MR motion artifact correction have been extensively studied. Although these approaches have shown high performance and reduced computational complexity compared to classical methods, most of them require supervised training using paired artifact-free and artifact-corrupted images, which may prohibit its use in many important clinical applications. For example, transient severe motion (TSM) due to acute transient dyspnea in Gd-EOB-DTPA-enhanced MR is difficult to control and model for paired data generation. To address this issue, here we propose a novel unsupervised deep learning scheme through outlier-rejecting bootstrap subsampling and aggregation. This is inspired by the observation that motions usually cause sparse k-space outliers in the phase encoding direction, so k-space subsampling along the phase encoding direction can remove some outliers and the aggregation step can further improve the results from the reconstruction network. Our method does not require any paired data because the training step only requires artifact-free images. Furthermore, to address the smoothing from potential bias to the artifact-free images, the network is trained in an unsupervised manner using optimal transport driven cycleGAN. We verify that our method can be applied for artifact correction from simulated motion as well as real motion from TSM successfully, outperforming existing state-of-the-art deep learning methods.
Unpaired Deep Learning for Accelerated MRI using Optimal Transport Driven CycleGAN
Aug 29, 2020

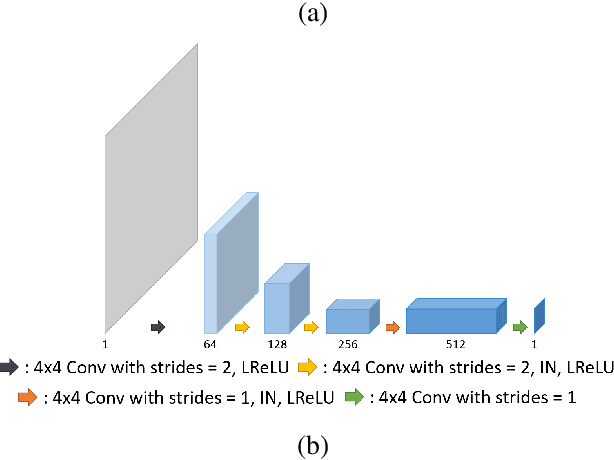
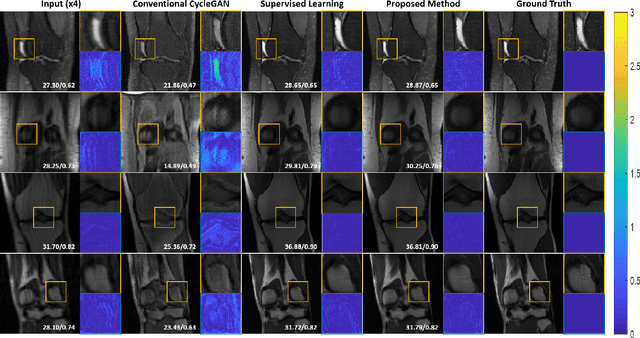
Abstract:Recently, deep learning approaches for accelerated MRI have been extensively studied thanks to their high performance reconstruction in spite of significantly reduced runtime complexity. These neural networks are usually trained in a supervised manner, so matched pairs of subsampled and fully sampled k-space data are required. Unfortunately, it is often difficult to acquire matched fully sampled k-space data, since the acquisition of fully sampled k-space data requires long scan time and often leads to the change of the acquisition protocol. Therefore, unpaired deep learning without matched label data has become a very important research topic. In this paper, we propose an unpaired deep learning approach using a optimal transport driven cycle-consistent generative adversarial network (OT-cycleGAN) that employs a single pair of generator and discriminator. The proposed OT-cycleGAN architecture is rigorously derived from a dual formulation of the optimal transport formulation using a specially designed penalized least squares cost. The experimental results show that our method can reconstruct high resolution MR images from accelerated k- space data from both single and multiple coil acquisition, without requiring matched reference data.
Geometric Approaches to Increase the Expressivity of Deep Neural Networks for MR Reconstruction
Mar 17, 2020



Abstract:Recently, deep learning approaches have been extensively investigated to reconstruct images from accelerated magnetic resonance image (MRI) acquisition. Although these approaches provide significant performance gain compared to compressed sensing MRI (CS-MRI), it is not clear how to choose a suitable network architecture to balance the trade-off between network complexity and performance. Recently, it was shown that an encoder-decoder convolutional neural network (CNN) can be interpreted as a piecewise linear basis-like representation, whose specific representation is determined by the ReLU activation patterns for a given input image. Thus, the expressivity or the representation power is determined by the number of piecewise linear regions. As an extension of this geometric understanding, this paper proposes a systematic geometric approach using bootstrapping and subnetwork aggregation using an attention module to increase the expressivity of the underlying neural network. Our method can be implemented in both k-space domain and image domain that can be trained in an end-to-end manner. Experimental results show that the proposed schemes significantly improve reconstruction performance with negligible complexity increases.
Optimal Transport, CycleGAN, and Penalized LS for Unsupervised Learning in Inverse Problems
Sep 25, 2019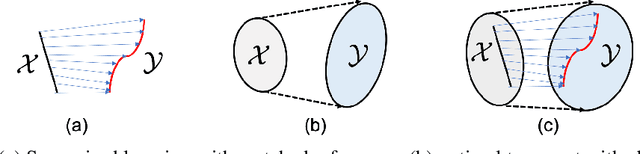
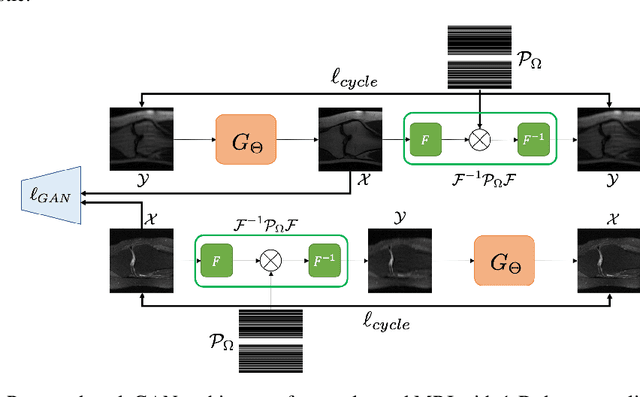

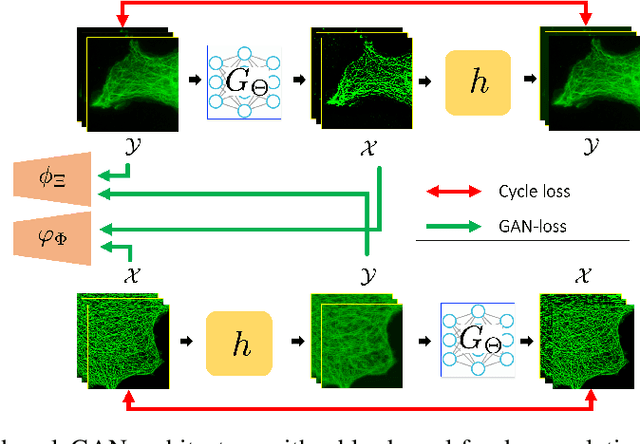
Abstract:The penalized least squares (PLS) is a classic approach to inverse problems, where a regularization term is added to stabilize the solution. Optimal transport (OT) is another mathematical framework for computer vision tasks by providing means to transport one measure to another at a minimal cost. Cycle-consistent generative adversarial network (cycleGAN) is a recent extension of GAN to learn target distributions with less mode collapsing behaviour. Although similar in that no supervised training is required, the algorithms look different, so the mathematical relationship between these approaches is not clear. In this article, we provide an important advance to unveil the missing link. Specifically, we reveal that a cycleGAN architecture can be derived as a dual formulation of the optimal transport problem, if the PLS with a deep learning penalty is used as a transport cost between the two probability measures from measurements and unknown images. This suggests that cycleGAN can be considered as a stochastic generalization of classical PLS approaches. Our derivation is so general that various types of cycleGAN architecture can be easily derived by merely changing the transport cost. As proofs of concept, this paper provides novel cycleGAN architecture for unsupervised learning in accelerated MRI and deconvolution microscopy problems, which confirm the efficacy and the flexibility of the theory.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge