Guoping Cai
WDFFU-Mamba: A Wavelet-guided Dual-attention Feature Fusion Mamba for Breast Tumor Segmentation in Ultrasound Images
Dec 19, 2025Abstract:Breast ultrasound (BUS) image segmentation plays a vital role in assisting clinical diagnosis and early tumor screening. However, challenges such as speckle noise, imaging artifacts, irregular lesion morphology, and blurred boundaries severely hinder accurate segmentation. To address these challenges, this work aims to design a robust and efficient model capable of automatically segmenting breast tumors in BUS images.We propose a novel segmentation network named WDFFU-Mamba, which integrates wavelet-guided enhancement and dual-attention feature fusion within a U-shaped Mamba architecture. A Wavelet-denoised High-Frequency-guided Feature (WHF) module is employed to enhance low-level representations through noise-suppressed high-frequency cues. A Dual Attention Feature Fusion (DAFF) module is also introduced to effectively merge skip-connected and semantic features, improving contextual consistency.Extensive experiments on two public BUS datasets demonstrate that WDFFU-Mamba achieves superior segmentation accuracy, significantly outperforming existing methods in terms of Dice coefficient and 95th percentile Hausdorff Distance (HD95).The combination of wavelet-domain enhancement and attention-based fusion greatly improves both the accuracy and robustness of BUS image segmentation, while maintaining computational efficiency.The proposed WDFFU-Mamba model not only delivers strong segmentation performance but also exhibits desirable generalization ability across datasets, making it a promising solution for real-world clinical applications in breast tumor ultrasound analysis.
Pathologist-like explainable AI for interpretable Gleason grading in prostate cancer
Oct 19, 2024



Abstract:The aggressiveness of prostate cancer, the most common cancer in men worldwide, is primarily assessed based on histopathological data using the Gleason scoring system. While artificial intelligence (AI) has shown promise in accurately predicting Gleason scores, these predictions often lack inherent explainability, potentially leading to distrust in human-machine interactions. To address this issue, we introduce a novel dataset of 1,015 tissue microarray core images, annotated by an international group of 54 pathologists. The annotations provide detailed localized pattern descriptions for Gleason grading in line with international guidelines. Utilizing this dataset, we develop an inherently explainable AI system based on a U-Net architecture that provides predictions leveraging pathologists' terminology. This approach circumvents post-hoc explainability methods while maintaining or exceeding the performance of methods trained directly for Gleason pattern segmentation (Dice score: 0.713 $\pm$ 0.003 trained on explanations vs. 0.691 $\pm$ 0.010 trained on Gleason patterns). By employing soft labels during training, we capture the intrinsic uncertainty in the data, yielding strong results in Gleason pattern segmentation even in the context of high interobserver variability. With the release of this dataset, we aim to encourage further research into segmentation in medical tasks with high levels of subjectivity and to advance the understanding of pathologists' reasoning processes.
Control with Distributed Deep Reinforcement Learning: Learn a Better Policy
Nov 30, 2018



Abstract:Distributed approach is a very effective method to improve training efficiency of reinforcement learning. In this paper, we propose a new heuristic distributed architecture for deep reinforcement learning (DRL) algorithm, in which a PSO based network update mechanism is adopted to speed up learning an optimal policy besides using multiple agents for parallel training. In this mechanism, the update of neural network of each agent is not only according to the training result of itself, but also affected by the optimal neural network of all agents. In order to verify the effectiveness of the proposed method, the proposed architecture is implemented on the Deep Q-Network algorithm (DQN) and the Deep Deterministic Policy Gradient algorithm (DDPG) to train several typical control problems. The training results show that the proposed method is effective.
Computer-aided diagnosis of lung carcinoma using deep learning - a pilot study
Mar 14, 2018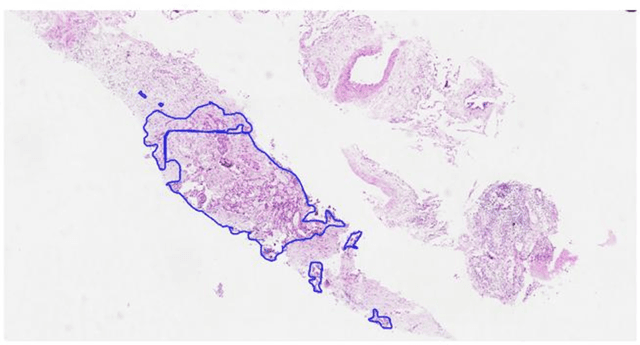
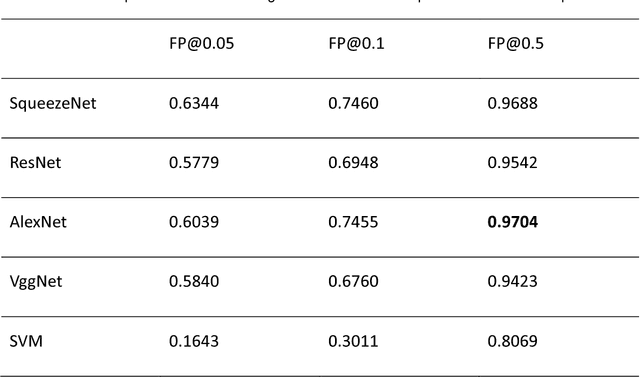
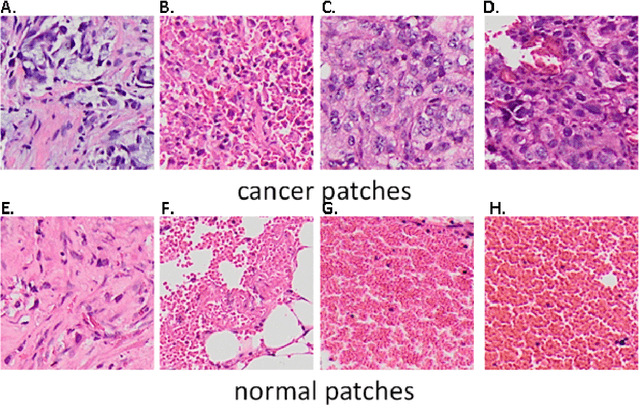
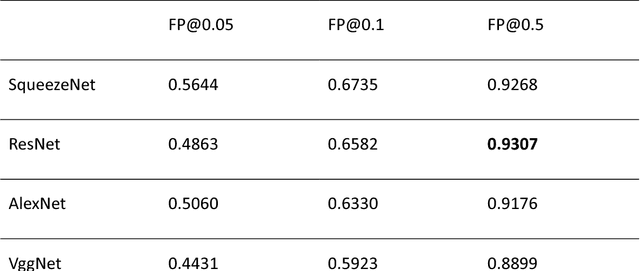
Abstract:Aim: Early detection and correct diagnosis of lung cancer are the most important steps in improving patient outcome. This study aims to assess which deep learning models perform best in lung cancer diagnosis. Methods: Non-small cell lung carcinoma and small cell lung carcinoma biopsy specimens were consecutively obtained and stained. The specimen slides were diagnosed by two experienced pathologists (over 20 years). Several deep learning models were trained to discriminate cancer and non-cancer biopsies. Result: Deep learning models give reasonable AUC from 0.8810 to 0.9119. Conclusion: The deep learning analysis could help to speed up the detection process for the whole-slide image (WSI) and keep the comparable detection rate with human observer.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge