Sarah Haggenmüller
Pathologist-like explainable AI for interpretable Gleason grading in prostate cancer
Oct 19, 2024



Abstract:The aggressiveness of prostate cancer, the most common cancer in men worldwide, is primarily assessed based on histopathological data using the Gleason scoring system. While artificial intelligence (AI) has shown promise in accurately predicting Gleason scores, these predictions often lack inherent explainability, potentially leading to distrust in human-machine interactions. To address this issue, we introduce a novel dataset of 1,015 tissue microarray core images, annotated by an international group of 54 pathologists. The annotations provide detailed localized pattern descriptions for Gleason grading in line with international guidelines. Utilizing this dataset, we develop an inherently explainable AI system based on a U-Net architecture that provides predictions leveraging pathologists' terminology. This approach circumvents post-hoc explainability methods while maintaining or exceeding the performance of methods trained directly for Gleason pattern segmentation (Dice score: 0.713 $\pm$ 0.003 trained on explanations vs. 0.691 $\pm$ 0.010 trained on Gleason patterns). By employing soft labels during training, we capture the intrinsic uncertainty in the data, yielding strong results in Gleason pattern segmentation even in the context of high interobserver variability. With the release of this dataset, we aim to encourage further research into segmentation in medical tasks with high levels of subjectivity and to advance the understanding of pathologists' reasoning processes.
Superhuman performance in urology board questions by an explainable large language model enabled for context integration of the European Association of Urology guidelines: the UroBot study
Jun 04, 2024



Abstract:Large Language Models (LLMs) are revolutionizing medical Question-Answering (medQA) through extensive use of medical literature. However, their performance is often hampered by outdated training data and a lack of explainability, which limits clinical applicability. This study aimed to create and assess UroBot, a urology-specialized chatbot, by comparing it with state-of-the-art models and the performance of urologists on urological board questions, ensuring full clinician-verifiability. UroBot was developed using OpenAI's GPT-3.5, GPT-4, and GPT-4o models, employing retrieval-augmented generation (RAG) and the latest 2023 guidelines from the European Association of Urology (EAU). The evaluation included ten runs of 200 European Board of Urology (EBU) In-Service Assessment (ISA) questions, with performance assessed by the mean Rate of Correct Answers (RoCA). UroBot-4o achieved an average RoCA of 88.4%, surpassing GPT-4o by 10.8%, with a score of 77.6%. It was also clinician-verifiable and exhibited the highest run agreement as indicated by Fleiss' Kappa (k = 0.979). By comparison, the average performance of urologists on board questions, as reported in the literature, is 68.7%. UroBot's clinician-verifiable nature and superior accuracy compared to both existing models and urologists on board questions highlight its potential for clinical integration. The study also provides the necessary code and instructions for further development of UroBot.
Advancing dermatological diagnosis: Development of a hyperspectral dermatoscope for enhanced skin imaging
Mar 01, 2024



Abstract:Clinical dermatology necessitates precision and innovation for efficient diagnosis and treatment of various skin conditions. This paper introduces the development of a cutting-edge hyperspectral dermatoscope (the Hyperscope) tailored for human skin analysis. We detail the requirements to such a device and the design considerations, from optical configurations to sensor selection, necessary to capture a wide spectral range with high fidelity. Preliminary results from 15 individuals and 160 recorded skin images demonstrate the potential of the Hyperscope in identifying and characterizing various skin conditions, offering a promising avenue for non-invasive skin evaluation and a platform for future research in dermatology-related hyperspectral imaging.
Clinical Melanoma Diagnosis with Artificial Intelligence: Insights from a Prospective Multicenter Study
Jan 25, 2024Abstract:Early detection of melanoma, a potentially lethal type of skin cancer with high prevalence worldwide, improves patient prognosis. In retrospective studies, artificial intelligence (AI) has proven to be helpful for enhancing melanoma detection. However, there are few prospective studies confirming these promising results. Existing studies are limited by low sample sizes, too homogenous datasets, or lack of inclusion of rare melanoma subtypes, preventing a fair and thorough evaluation of AI and its generalizability, a crucial aspect for its application in the clinical setting. Therefore, we assessed 'All Data are Ext' (ADAE), an established open-source ensemble algorithm for detecting melanomas, by comparing its diagnostic accuracy to that of dermatologists on a prospectively collected, external, heterogeneous test set comprising eight distinct hospitals, four different camera setups, rare melanoma subtypes, and special anatomical sites. We advanced the algorithm with real test-time augmentation (R-TTA, i.e. providing real photographs of lesions taken from multiple angles and averaging the predictions), and evaluated its generalization capabilities. Overall, the AI showed higher balanced accuracy than dermatologists (0.798, 95% confidence interval (CI) 0.779-0.814 vs. 0.781, 95% CI 0.760-0.802; p<0.001), obtaining a higher sensitivity (0.921, 95% CI 0.900- 0.942 vs. 0.734, 95% CI 0.701-0.770; p<0.001) at the cost of a lower specificity (0.673, 95% CI 0.641-0.702 vs. 0.828, 95% CI 0.804-0.852; p<0.001). As the algorithm exhibited a significant performance advantage on our heterogeneous dataset exclusively comprising melanoma-suspicious lesions, AI may offer the potential to support dermatologists particularly in diagnosing challenging cases.
Using Multiple Dermoscopic Photographs of One Lesion Improves Melanoma Classification via Deep Learning: A Prognostic Diagnostic Accuracy Study
Jun 05, 2023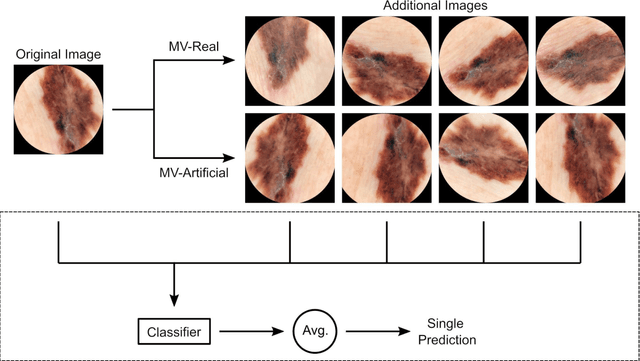
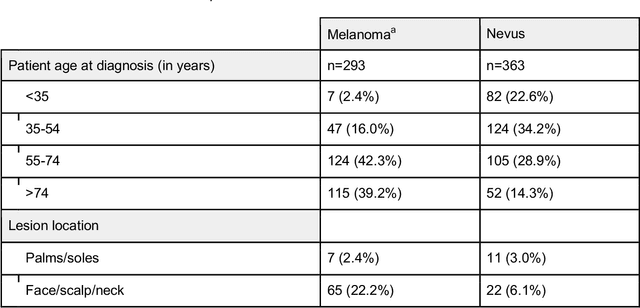
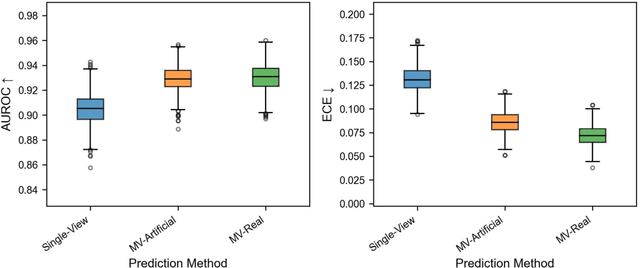
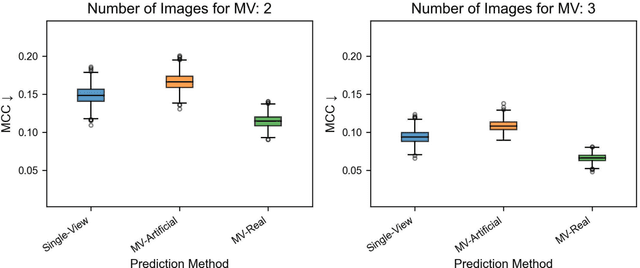
Abstract:Background: Convolutional neural network (CNN)-based melanoma classifiers face several challenges that limit their usefulness in clinical practice. Objective: To investigate the impact of multiple real-world dermoscopic views of a single lesion of interest on a CNN-based melanoma classifier. Methods: This study evaluated 656 suspected melanoma lesions. Classifier performance was measured using area under the receiver operating characteristic curve (AUROC), expected calibration error (ECE) and maximum confidence change (MCC) for (I) a single-view scenario, (II) a multiview scenario using multiple artificially modified images per lesion and (III) a multiview scenario with multiple real-world images per lesion. Results: The multiview approach with real-world images significantly increased the AUROC from 0.905 (95% CI, 0.879-0.929) in the single-view approach to 0.930 (95% CI, 0.909-0.951). ECE and MCC also improved significantly from 0.131 (95% CI, 0.105-0.159) to 0.072 (95% CI: 0.052-0.093) and from 0.149 (95% CI, 0.125-0.171) to 0.115 (95% CI: 0.099-0.131), respectively. Comparing multiview real-world to artificially modified images showed comparable diagnostic accuracy and uncertainty estimation, but significantly worse robustness for the latter. Conclusion: Using multiple real-world images is an inexpensive method to positively impact the performance of a CNN-based melanoma classifier.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge