Lukas Heinlein
A Multivocal Literature Review on Privacy and Fairness in Federated Learning
Aug 16, 2024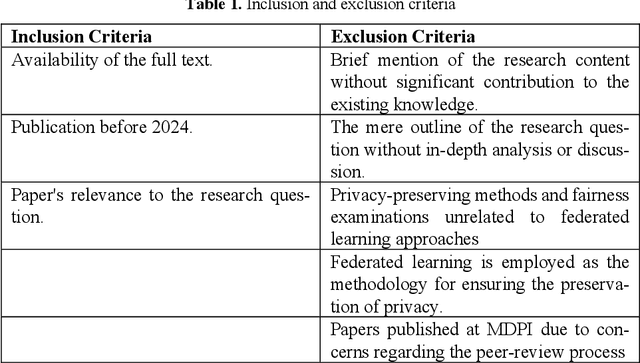
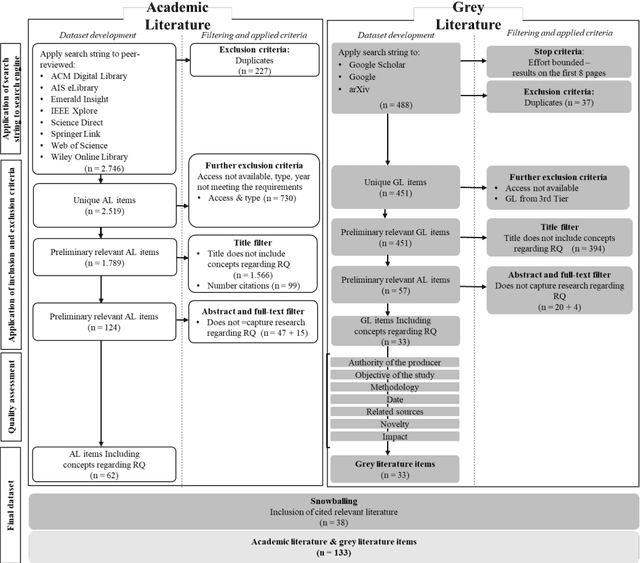
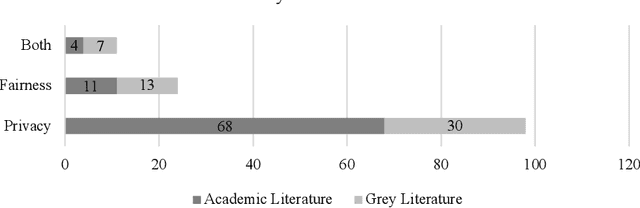
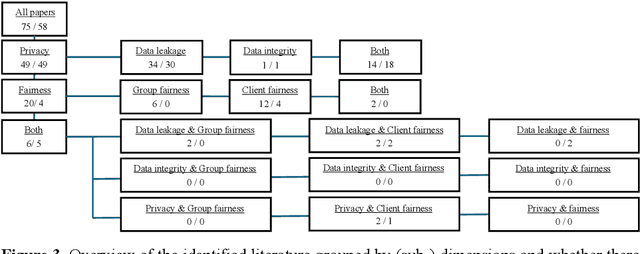
Abstract:Federated Learning presents a way to revolutionize AI applications by eliminating the necessity for data sharing. Yet, research has shown that information can still be extracted during training, making additional privacy-preserving measures such as differential privacy imperative. To implement real-world federated learning applications, fairness, ranging from a fair distribution of performance to non-discriminative behaviour, must be considered. Particularly in high-risk applications (e.g. healthcare), avoiding the repetition of past discriminatory errors is paramount. As recent research has demonstrated an inherent tension between privacy and fairness, we conduct a multivocal literature review to examine the current methods to integrate privacy and fairness in federated learning. Our analyses illustrate that the relationship between privacy and fairness has been neglected, posing a critical risk for real-world applications. We highlight the need to explore the relationship between privacy, fairness, and performance, advocating for the creation of integrated federated learning frameworks.
Clinical Melanoma Diagnosis with Artificial Intelligence: Insights from a Prospective Multicenter Study
Jan 25, 2024Abstract:Early detection of melanoma, a potentially lethal type of skin cancer with high prevalence worldwide, improves patient prognosis. In retrospective studies, artificial intelligence (AI) has proven to be helpful for enhancing melanoma detection. However, there are few prospective studies confirming these promising results. Existing studies are limited by low sample sizes, too homogenous datasets, or lack of inclusion of rare melanoma subtypes, preventing a fair and thorough evaluation of AI and its generalizability, a crucial aspect for its application in the clinical setting. Therefore, we assessed 'All Data are Ext' (ADAE), an established open-source ensemble algorithm for detecting melanomas, by comparing its diagnostic accuracy to that of dermatologists on a prospectively collected, external, heterogeneous test set comprising eight distinct hospitals, four different camera setups, rare melanoma subtypes, and special anatomical sites. We advanced the algorithm with real test-time augmentation (R-TTA, i.e. providing real photographs of lesions taken from multiple angles and averaging the predictions), and evaluated its generalization capabilities. Overall, the AI showed higher balanced accuracy than dermatologists (0.798, 95% confidence interval (CI) 0.779-0.814 vs. 0.781, 95% CI 0.760-0.802; p<0.001), obtaining a higher sensitivity (0.921, 95% CI 0.900- 0.942 vs. 0.734, 95% CI 0.701-0.770; p<0.001) at the cost of a lower specificity (0.673, 95% CI 0.641-0.702 vs. 0.828, 95% CI 0.804-0.852; p<0.001). As the algorithm exhibited a significant performance advantage on our heterogeneous dataset exclusively comprising melanoma-suspicious lesions, AI may offer the potential to support dermatologists particularly in diagnosing challenging cases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge