Cheng Ye
EmoVerse: A MLLMs-Driven Emotion Representation Dataset for Interpretable Visual Emotion Analysis
Nov 16, 2025
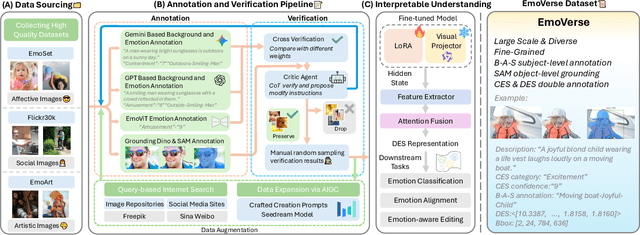
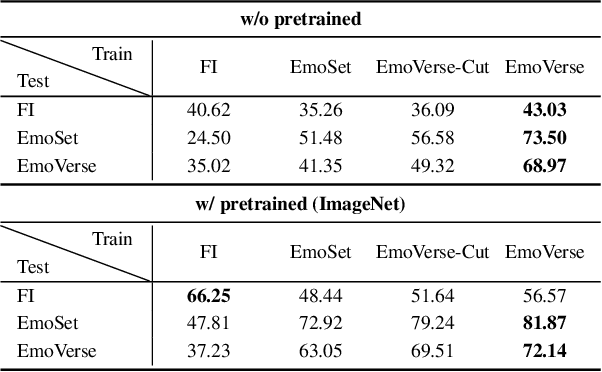
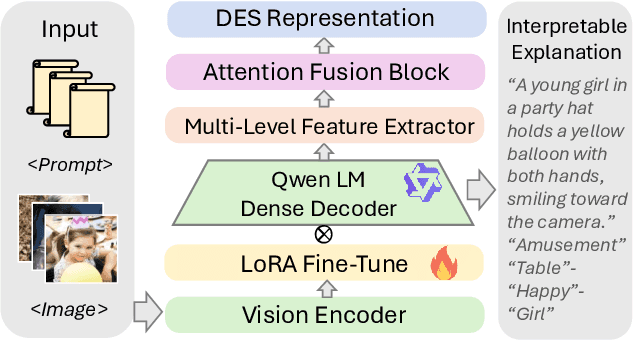
Abstract:Visual Emotion Analysis (VEA) aims to bridge the affective gap between visual content and human emotional responses. Despite its promise, progress in this field remains limited by the lack of open-source and interpretable datasets. Most existing studies assign a single discrete emotion label to an entire image, offering limited insight into how visual elements contribute to emotion. In this work, we introduce EmoVerse, a large-scale open-source dataset that enables interpretable visual emotion analysis through multi-layered, knowledge-graph-inspired annotations. By decomposing emotions into Background-Attribute-Subject (B-A-S) triplets and grounding each element to visual regions, EmoVerse provides word-level and subject-level emotional reasoning. With over 219k images, the dataset further includes dual annotations in Categorical Emotion States (CES) and Dimensional Emotion Space (DES), facilitating unified discrete and continuous emotion representation. A novel multi-stage pipeline ensures high annotation reliability with minimal human effort. Finally, we introduce an interpretable model that maps visual cues into DES representations and provides detailed attribution explanations. Together, the dataset, pipeline, and model form a comprehensive foundation for advancing explainable high-level emotion understanding.
Dual-path Collaborative Generation Network for Emotional Video Captioning
Aug 06, 2024



Abstract:Emotional Video Captioning is an emerging task that aims to describe factual content with the intrinsic emotions expressed in videos. The essential of the EVC task is to effectively perceive subtle and ambiguous visual emotional cues during the caption generation, which is neglected by the traditional video captioning. Existing emotional video captioning methods perceive global visual emotional cues at first, and then combine them with the video features to guide the emotional caption generation, which neglects two characteristics of the EVC task. Firstly, their methods neglect the dynamic subtle changes in the intrinsic emotions of the video, which makes it difficult to meet the needs of common scenes with diverse and changeable emotions. Secondly, as their methods incorporate emotional cues into each step, the guidance role of emotion is overemphasized, which makes factual content more or less ignored during generation. To this end, we propose a dual-path collaborative generation network, which dynamically perceives visual emotional cues evolutions while generating emotional captions by collaborative learning. Specifically, in the dynamic emotion perception path, we propose a dynamic emotion evolution module, which first aggregates visual features and historical caption features to summarize the global visual emotional cues, and then dynamically selects emotional cues required to be re-composed at each stage. Besides, in the adaptive caption generation path, to balance the description of factual content and emotional cues, we propose an emotion adaptive decoder. Thus, our methods can generate emotion-related words at the necessary time step, and our caption generation balances the guidance of factual content and emotional cues well. Extensive experiments on three challenging datasets demonstrate the superiority of our approach and each proposed module.
Understanding the Performance of Knowledge Graph Embeddings in Drug Discovery
Jun 07, 2021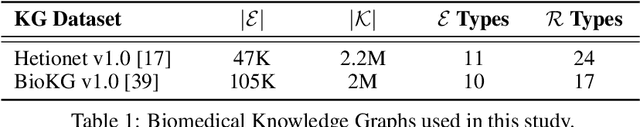
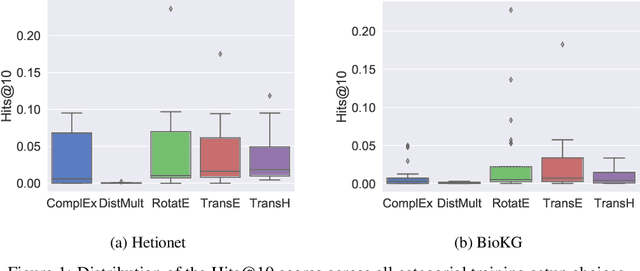
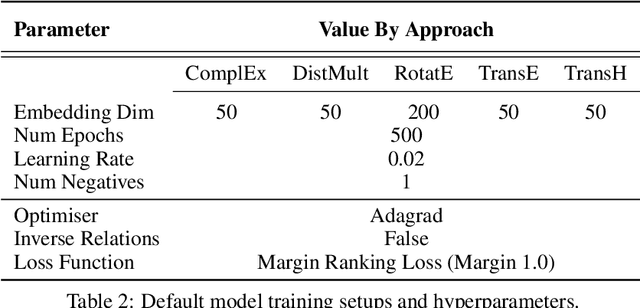
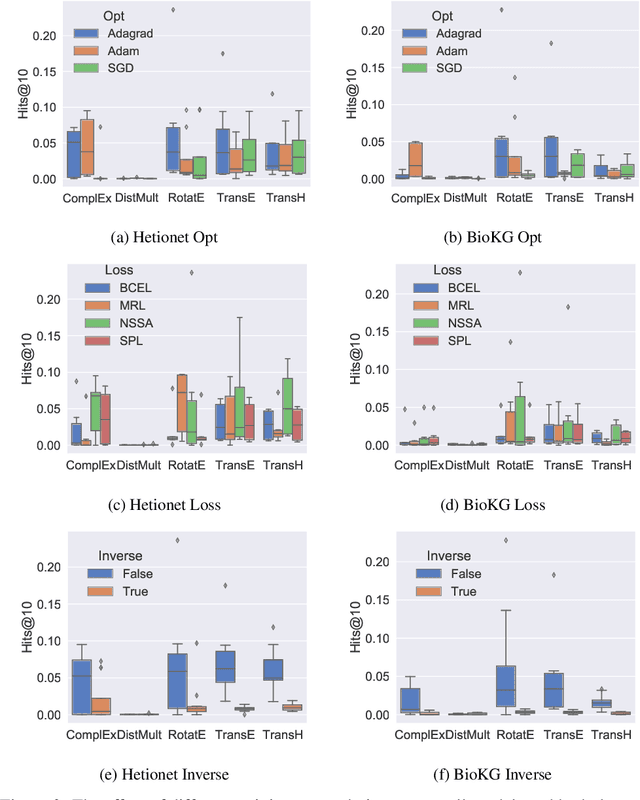
Abstract:Knowledge Graphs (KG) and associated Knowledge Graph Embedding (KGE) models have recently begun to be explored in the context of drug discovery and have the potential to assist in key challenges such as target identification. In the drug discovery domain, KGs can be employed as part of a process which can result in lab-based experiments being performed, or impact on other decisions, incurring significant time and financial costs and most importantly, ultimately influencing patient healthcare. For KGE models to have impact in this domain, a better understanding of not only of performance, but also the various factors which determine it, is required. In this study we investigate, over the course of many thousands of experiments, the predictive performance of five KGE models on two public drug discovery-oriented KGs. Our goal is not to focus on the best overall model or configuration, instead we take a deeper look at how performance can be affected by changes in the training setup, choice of hyperparameters, model parameter initialisation seed and different splits of the datasets. Our results highlight that these factors have significant impact on performance and can even affect the ranking of models. Indeed these factors should be reported along with model architectures to ensure complete reproducibility and fair comparisons of future work, and we argue this is critical for the acceptance of use, and impact of KGEs in a biomedical setting. To aid reproducibility of our own work, we release all experimentation code.
Predicting Potential Drug Targets Using Tensor Factorisation and Knowledge Graph Embeddings
May 20, 2021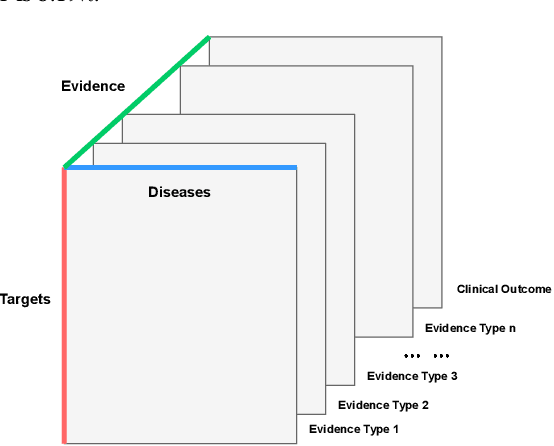
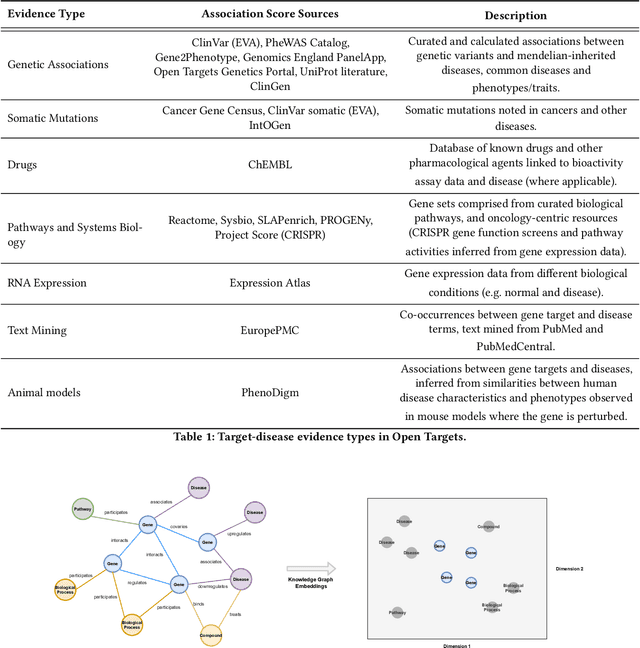
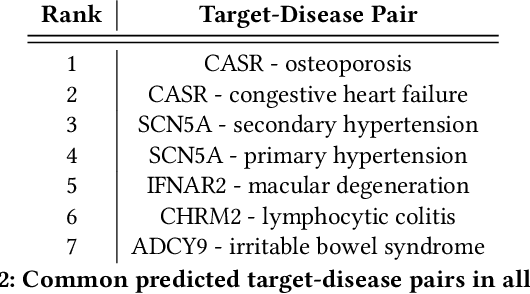
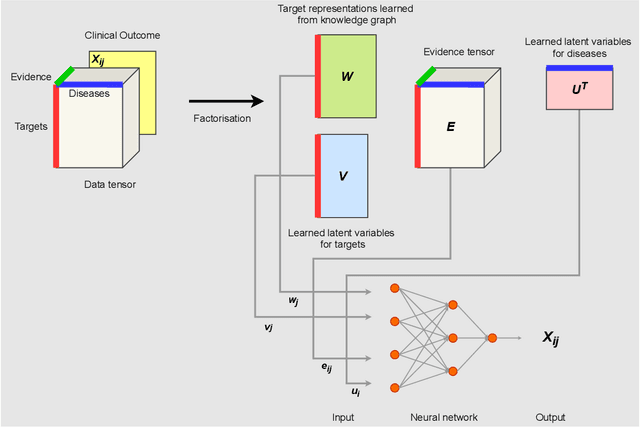
Abstract:The drug discovery and development process is a long and expensive one, costing over 1 billion USD on average per drug and taking 10-15 years. To reduce the high levels of attrition throughout the process, there has been a growing interest in applying machine learning methodologies to various stages of drug discovery process in the recent decade, including at the earliest stage - identification of druggable disease genes. In this paper, we have developed a new tensor factorisation model to predict potential drug targets (i.e.,genes or proteins) for diseases. We created a three dimensional tensor which consists of 1,048 targets, 860 diseases and 230,011 evidence attributes and clinical outcomes connecting them, using data extracted from the Open Targets and PharmaProjects databases. We enriched the data with gene representations learned from a drug discovery-oriented knowledge graph and applied our proposed method to predict the clinical outcomes for unseen target and dis-ease pairs. We designed three evaluation strategies to measure the prediction performance and benchmarked several commonly used machine learning classifiers together with matrix and tensor factorisation methods. The result shows that incorporating knowledge graph embeddings significantly improves the prediction accuracy and that training tensor factorisation alongside a dense neural network outperforms other methods. In summary, our framework combines two actively studied machine learning approaches to disease target identification, tensor factorisation and knowledge graph representation learning, which could be a promising avenue for further exploration in data-driven drug discovery.
A Review of Biomedical Datasets Relating to Drug Discovery: A Knowledge Graph Perspective
Feb 26, 2021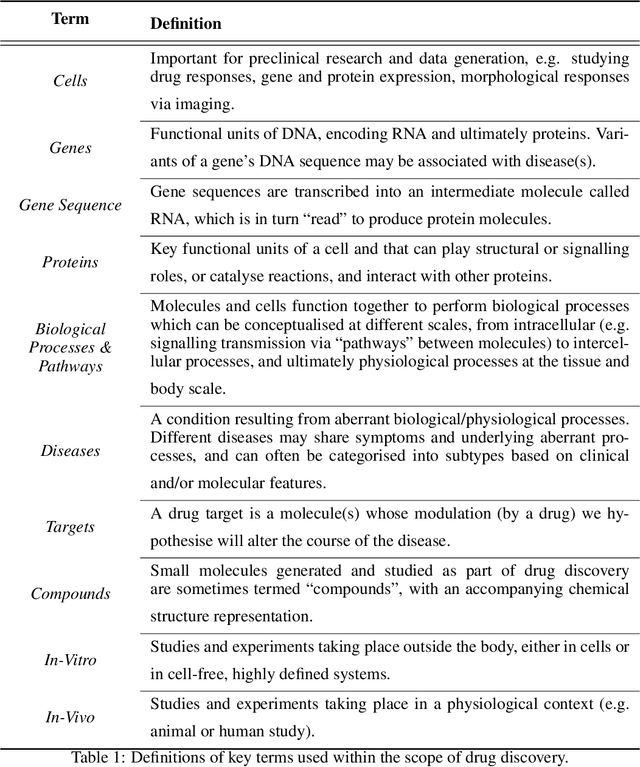
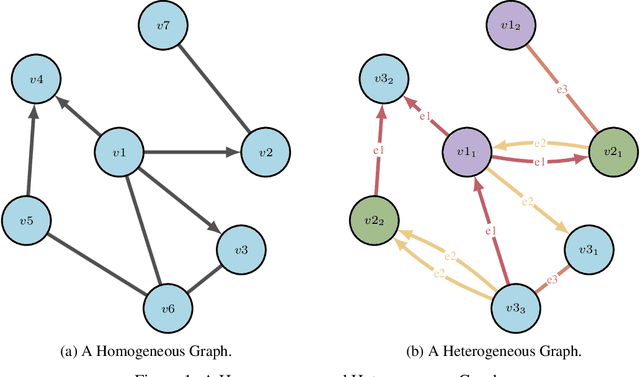
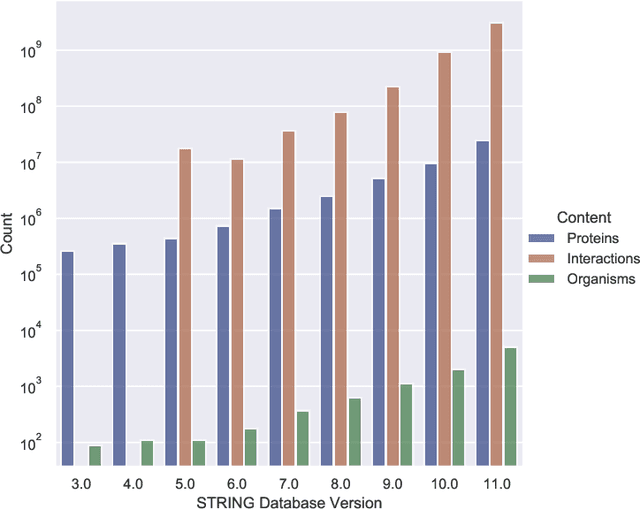
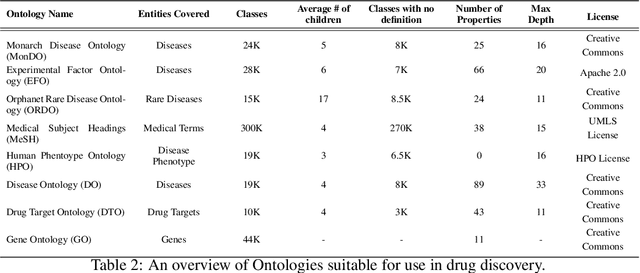
Abstract:Drug discovery and development is an extremely complex process, with high attrition contributing to the costs of delivering new medicines to patients. Recently, various machine learning approaches have been proposed and investigated to help improve the effectiveness and speed of multiple stages of the drug discovery pipeline. Among these techniques, it is especially those using Knowledge Graphs that are proving to have considerable promise across a range of tasks, including drug repurposing, drug toxicity prediction and target gene-disease prioritisation. In such a knowledge graph-based representation of drug discovery domains, crucial elements including genes, diseases and drugs are represented as entities or vertices, whilst relationships or edges between them indicate some level of interaction. For example, an edge between a disease and drug entity might represent a successful clinical trial, or an edge between two drug entities could indicate a potentially harmful interaction. In order to construct high-quality and ultimately informative knowledge graphs however, suitable data and information is of course required. In this review, we detail publicly available primary data sources containing information suitable for use in constructing various drug discovery focused knowledge graphs. We aim to help guide machine learning and knowledge graph practitioners who are interested in applying new techniques to the drug discovery field, but who may be unfamiliar with the relevant data sources. Overall we hope this review will help motivate more machine learning researchers to explore combining knowledge graphs and machine learning to help solve key and emerging questions in the drug discovery domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge