Ian P Barrett
The Role of Graph Topology in the Performance of Biomedical Knowledge Graph Completion Models
Sep 06, 2024



Abstract:Knowledge Graph Completion has been increasingly adopted as a useful method for several tasks in biomedical research, like drug repurposing or drug-target identification. To that end, a variety of datasets and Knowledge Graph Embedding models has been proposed over the years. However, little is known about the properties that render a dataset useful for a given task and, even though theoretical properties of Knowledge Graph Embedding models are well understood, their practical utility in this field remains controversial. We conduct a comprehensive investigation into the topological properties of publicly available biomedical Knowledge Graphs and establish links to the accuracy observed in real-world applications. By releasing all model predictions and a new suite of analysis tools we invite the community to build upon our work and continue improving the understanding of these crucial applications.
Implications of Topological Imbalance for Representation Learning on Biomedical Knowledge Graphs
Dec 13, 2021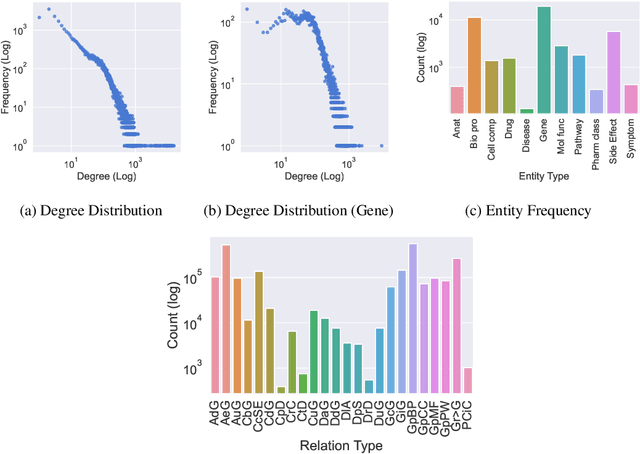
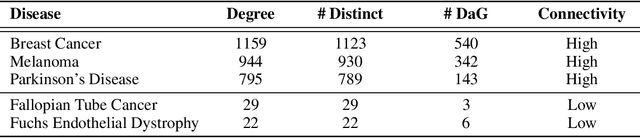
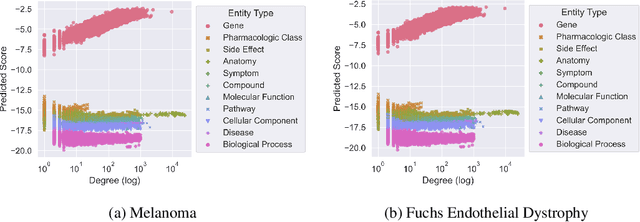
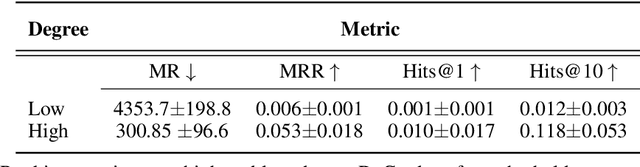
Abstract:Improving on the standard of care for diseases is predicated on better treatments, which in turn relies on finding and developing new drugs. However, drug discovery is a complex and costly process. Adoption of methods from machine learning has given rise to creation of drug discovery knowledge graphs which utilize the inherent interconnected nature of the domain. Graph-based data modelling, combined with knowledge graph embeddings provide a more intuitive representation of the domain and are suitable for inference tasks such as predicting missing links. One such example would be producing ranked lists of likely associated genes for a given disease, often referred to as target discovery. It is thus critical that these predictions are not only pertinent but also biologically meaningful. However, knowledge graphs can be biased either directly due to the underlying data sources that are integrated or due to modeling choices in the construction of the graph, one consequence of which is that certain entities can get topologically overrepresented. We show how knowledge graph embedding models can be affected by this structural imbalance, resulting in densely connected entities being highly ranked no matter the context. We provide support for this observation across different datasets, models and predictive tasks. Further, we show how the graph topology can be perturbed to artificially alter the rank of a gene via random, biologically meaningless information. This suggests that such models can be more influenced by the frequency of entities rather than biological information encoded in the relations, creating issues when entity frequency is not a true reflection of underlying data. Our results highlight the importance of data modeling choices and emphasizes the need for practitioners to be mindful of these issues when interpreting model outputs and during knowledge graph composition.
Understanding the Performance of Knowledge Graph Embeddings in Drug Discovery
Jun 07, 2021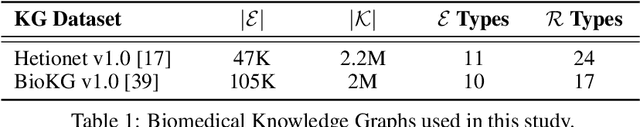
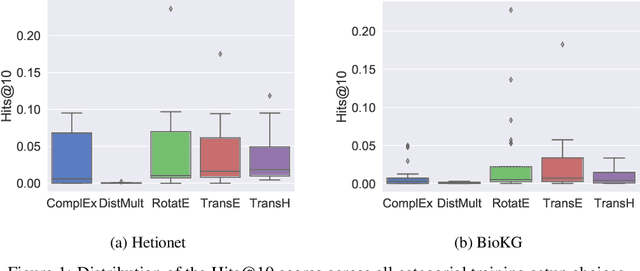
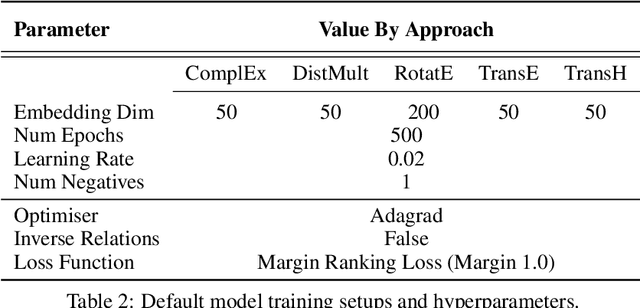
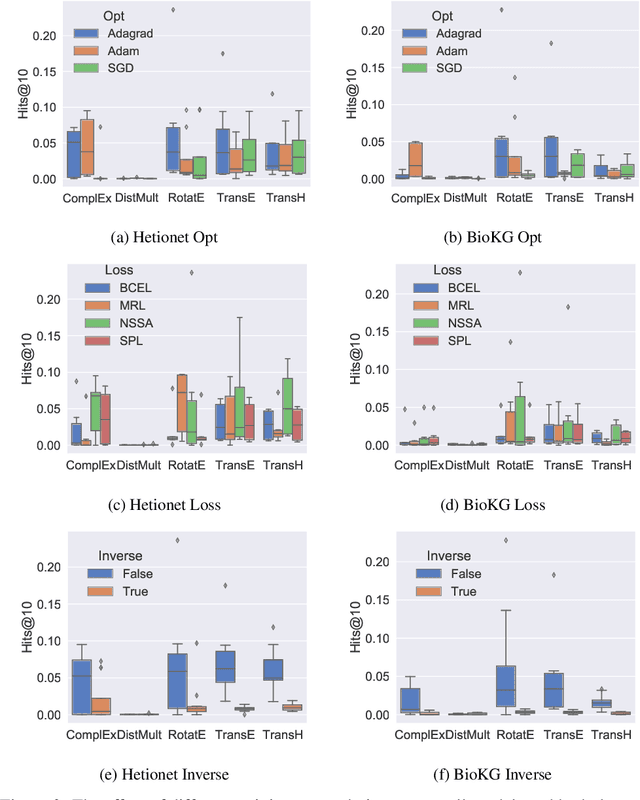
Abstract:Knowledge Graphs (KG) and associated Knowledge Graph Embedding (KGE) models have recently begun to be explored in the context of drug discovery and have the potential to assist in key challenges such as target identification. In the drug discovery domain, KGs can be employed as part of a process which can result in lab-based experiments being performed, or impact on other decisions, incurring significant time and financial costs and most importantly, ultimately influencing patient healthcare. For KGE models to have impact in this domain, a better understanding of not only of performance, but also the various factors which determine it, is required. In this study we investigate, over the course of many thousands of experiments, the predictive performance of five KGE models on two public drug discovery-oriented KGs. Our goal is not to focus on the best overall model or configuration, instead we take a deeper look at how performance can be affected by changes in the training setup, choice of hyperparameters, model parameter initialisation seed and different splits of the datasets. Our results highlight that these factors have significant impact on performance and can even affect the ranking of models. Indeed these factors should be reported along with model architectures to ensure complete reproducibility and fair comparisons of future work, and we argue this is critical for the acceptance of use, and impact of KGEs in a biomedical setting. To aid reproducibility of our own work, we release all experimentation code.
A Review of Biomedical Datasets Relating to Drug Discovery: A Knowledge Graph Perspective
Feb 26, 2021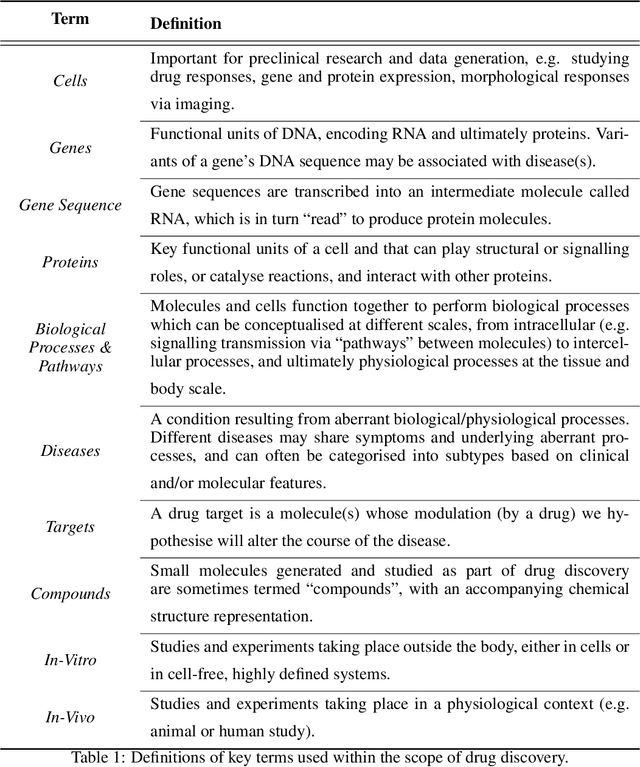
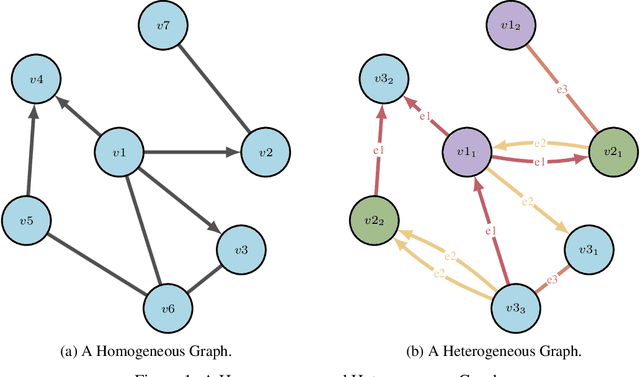
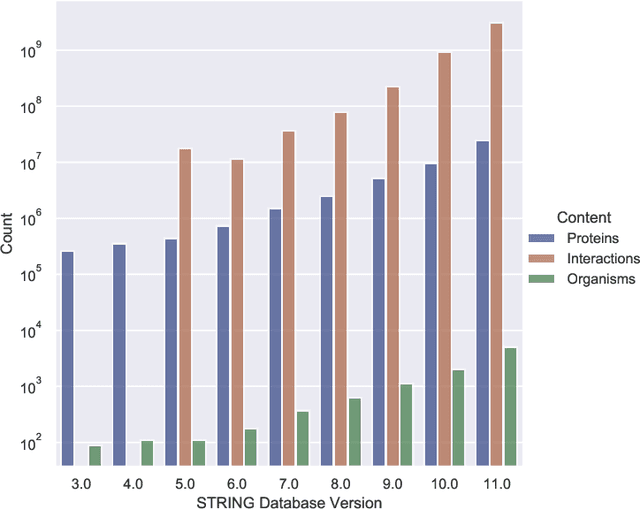
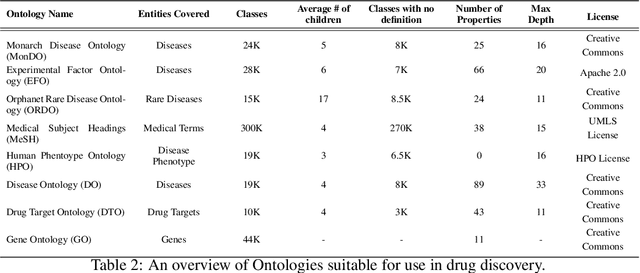
Abstract:Drug discovery and development is an extremely complex process, with high attrition contributing to the costs of delivering new medicines to patients. Recently, various machine learning approaches have been proposed and investigated to help improve the effectiveness and speed of multiple stages of the drug discovery pipeline. Among these techniques, it is especially those using Knowledge Graphs that are proving to have considerable promise across a range of tasks, including drug repurposing, drug toxicity prediction and target gene-disease prioritisation. In such a knowledge graph-based representation of drug discovery domains, crucial elements including genes, diseases and drugs are represented as entities or vertices, whilst relationships or edges between them indicate some level of interaction. For example, an edge between a disease and drug entity might represent a successful clinical trial, or an edge between two drug entities could indicate a potentially harmful interaction. In order to construct high-quality and ultimately informative knowledge graphs however, suitable data and information is of course required. In this review, we detail publicly available primary data sources containing information suitable for use in constructing various drug discovery focused knowledge graphs. We aim to help guide machine learning and knowledge graph practitioners who are interested in applying new techniques to the drug discovery field, but who may be unfamiliar with the relevant data sources. Overall we hope this review will help motivate more machine learning researchers to explore combining knowledge graphs and machine learning to help solve key and emerging questions in the drug discovery domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge