Anthony B Costa
BioNeMo Framework: a modular, high-performance library for AI model development in drug discovery
Nov 15, 2024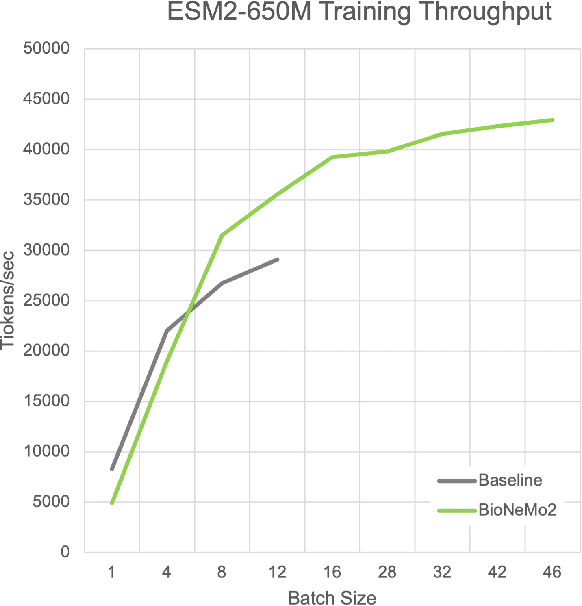
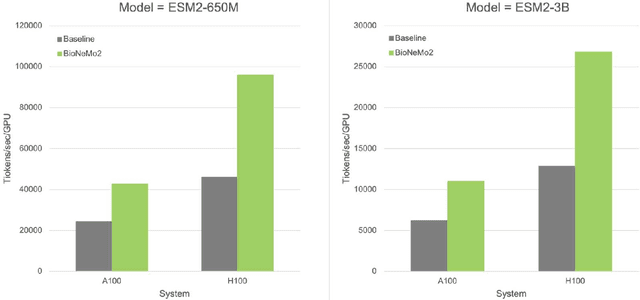
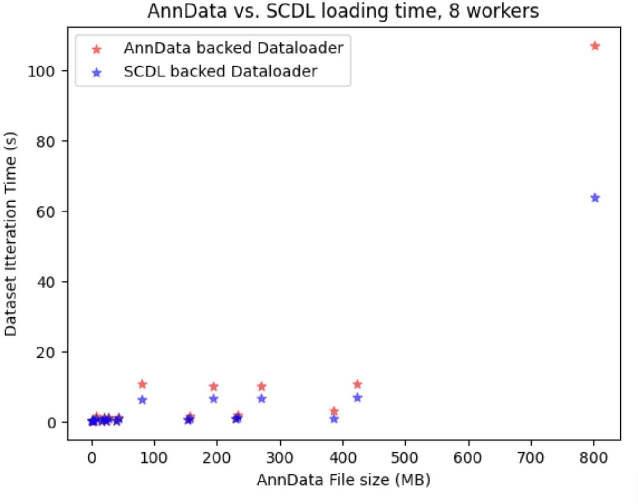
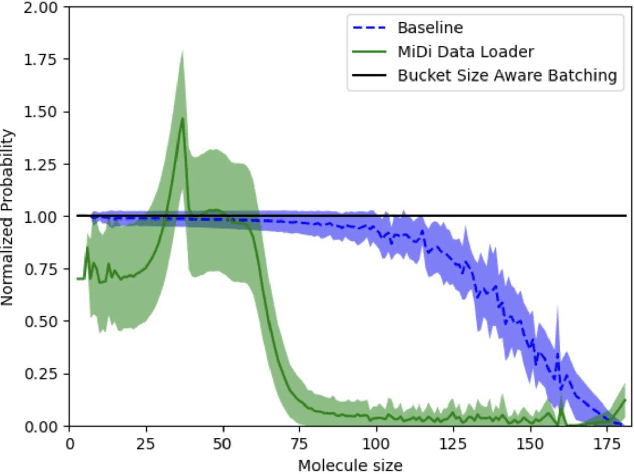
Abstract:Artificial Intelligence models encoding biology and chemistry are opening new routes to high-throughput and high-quality in-silico drug development. However, their training increasingly relies on computational scale, with recent protein language models (pLM) training on hundreds of graphical processing units (GPUs). We introduce the BioNeMo Framework to facilitate the training of computational biology and chemistry AI models across hundreds of GPUs. Its modular design allows the integration of individual components, such as data loaders, into existing workflows and is open to community contributions. We detail technical features of the BioNeMo Framework through use cases such as pLM pre-training and fine-tuning. On 256 NVIDIA A100s, BioNeMo Framework trains a three billion parameter BERT-based pLM on over one trillion tokens in 4.2 days. The BioNeMo Framework is open-source and free for everyone to use.
Generative Large Language Models Are All-purpose Text Analytics Engines: Text-to-text Learning Is All Your Need
Dec 11, 2023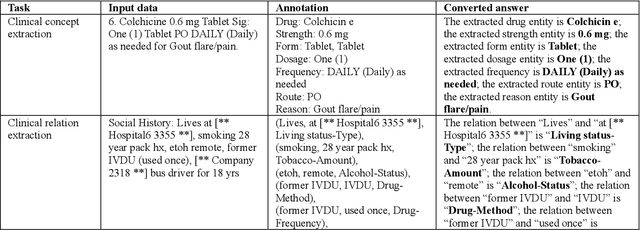
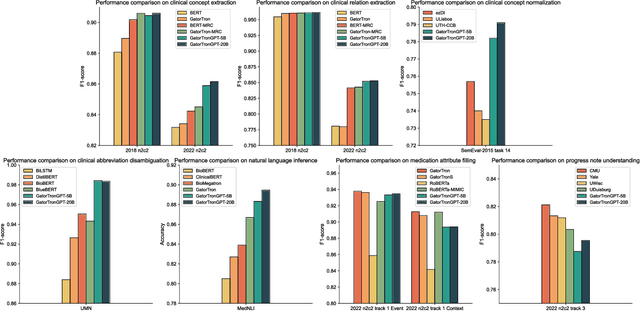
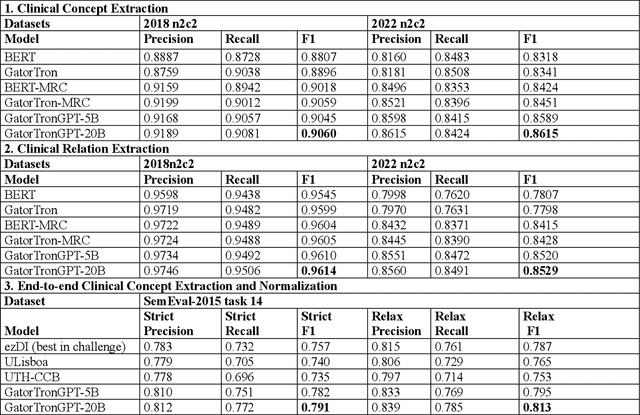
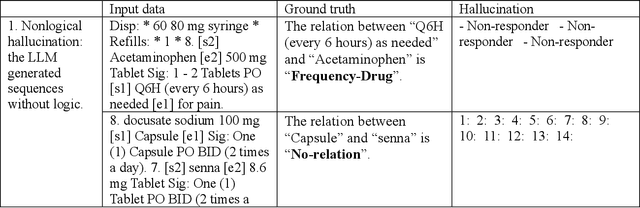
Abstract:Objective To solve major clinical natural language processing (NLP) tasks using a unified text-to-text learning architecture based on a generative large language model (LLM) via prompt tuning. Methods We formulated 7 key clinical NLP tasks as text-to-text learning and solved them using one unified generative clinical LLM, GatorTronGPT, developed using GPT-3 architecture and trained with up to 20 billion parameters. We adopted soft prompts (i.e., trainable vectors) with frozen LLM, where the LLM parameters were not updated (i.e., frozen) and only the vectors of soft prompts were updated, known as prompt tuning. We added additional soft prompts as a prefix to the input layer, which were optimized during the prompt tuning. We evaluated the proposed method using 7 clinical NLP tasks and compared them with previous task-specific solutions based on Transformer models. Results and Conclusion The proposed approach achieved state-of-the-art performance for 5 out of 7 major clinical NLP tasks using one unified generative LLM. Our approach outperformed previous task-specific transformer models by ~3% for concept extraction and 7% for relation extraction applied to social determinants of health, 3.4% for clinical concept normalization, 3.4~10% for clinical abbreviation disambiguation, and 5.5~9% for natural language inference. Our approach also outperformed a previously developed prompt-based machine reading comprehension (MRC) model, GatorTron-MRC, for clinical concept and relation extraction. The proposed approach can deliver the ``one model for all`` promise from training to deployment using a unified generative LLM.
A Study of Generative Large Language Model for Medical Research and Healthcare
May 22, 2023Abstract:There is enormous enthusiasm and concerns in using large language models (LLMs) in healthcare, yet current assumptions are all based on general-purpose LLMs such as ChatGPT. This study develops a clinical generative LLM, GatorTronGPT, using 277 billion words of mixed clinical and English text with a GPT-3 architecture of 20 billion parameters. GatorTronGPT improves biomedical natural language processing for medical research. Synthetic NLP models trained using GatorTronGPT generated text outperform NLP models trained using real-world clinical text. Physicians Turing test using 1 (worst) to 9 (best) scale shows that there is no significant difference in linguistic readability (p = 0.22; 6.57 of GatorTronGPT compared with 6.93 of human) and clinical relevance (p = 0.91; 7.0 of GatorTronGPT compared with 6.97 of human) and that physicians cannot differentiate them (p < 0.001). This study provides insights on the opportunities and challenges of LLMs for medical research and healthcare.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge