Alexander Derry
Prospector Heads: Generalized Feature Attribution for Large Models & Data
Feb 18, 2024Abstract:Feature attribution, the ability to localize regions of the input data that are relevant for classification, is an important capability for machine learning models in scientific and biomedical domains. Current methods for feature attribution, which rely on "explaining" the predictions of end-to-end classifiers, suffer from imprecise feature localization and are inadequate for use with small sample sizes and high-dimensional datasets due to computational challenges. We introduce prospector heads, an efficient and interpretable alternative to explanation-based methods for feature attribution that can be applied to any encoder and any data modality. Prospector heads generalize across modalities through experiments on sequences (text), images (pathology), and graphs (protein structures), outperforming baseline attribution methods by up to 49 points in mean localization AUPRC. We also demonstrate how prospector heads enable improved interpretation and discovery of class-specific patterns in the input data. Through their high performance, flexibility, and generalizability, prospectors provide a framework for improving trust and transparency for machine learning models in complex domains.
ATOM3D: Tasks On Molecules in Three Dimensions
Dec 07, 2020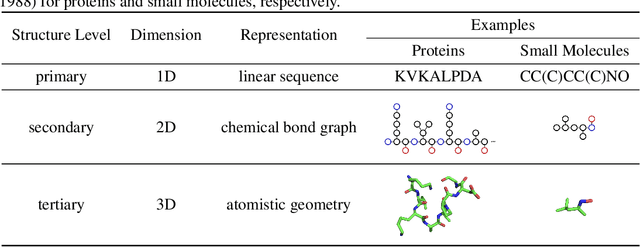
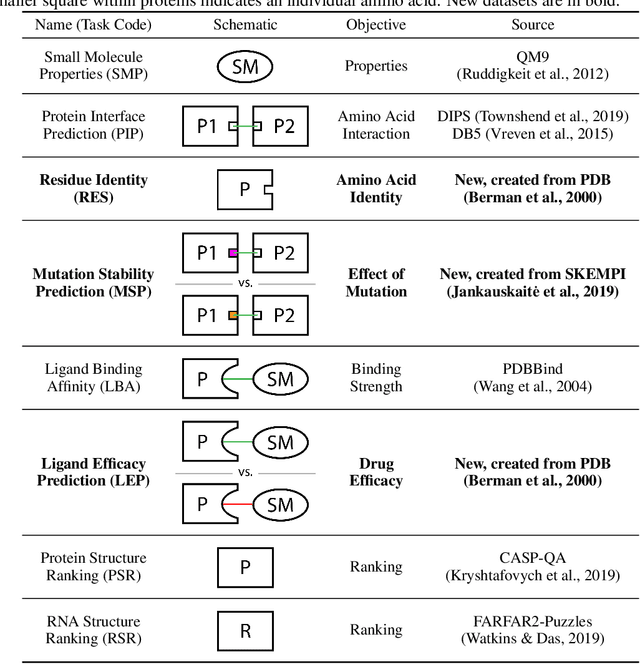
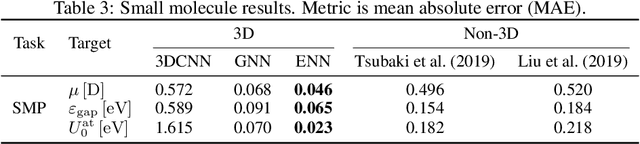
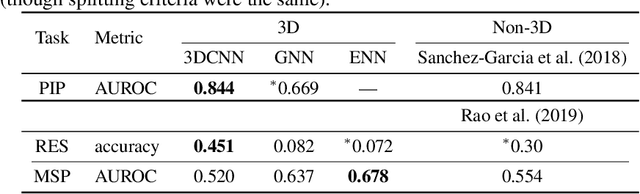
Abstract:Computational methods that operate directly on three-dimensional molecular structure hold large potential to solve important questions in biology and chemistry. In particular deep neural networks have recently gained significant attention. In this work we present ATOM3D, a collection of both novel and existing datasets spanning several key classes of biomolecules, to systematically assess such learning methods. We develop three-dimensional molecular learning networks for each of these tasks, finding that they consistently improve performance relative to one- and two-dimensional methods. The specific choice of architecture proves to be critical for performance, with three-dimensional convolutional networks excelling at tasks involving complex geometries, while graph networks perform well on systems requiring detailed positional information. Furthermore, equivariant networks show significant promise. Our results indicate many molecular problems stand to gain from three-dimensional molecular learning. All code and datasets can be accessed via https://www.atom3d.ai .
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge