Zhanwei Xu
The Medical Segmentation Decathlon
Jun 10, 2021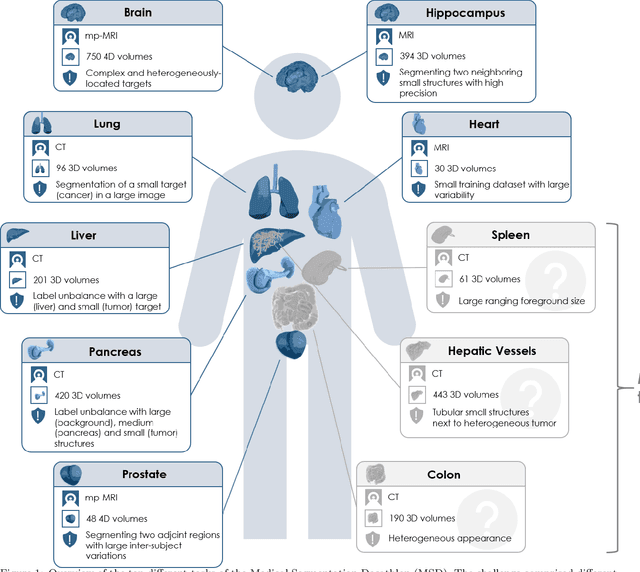
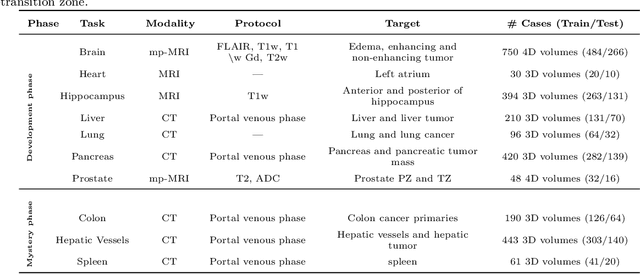
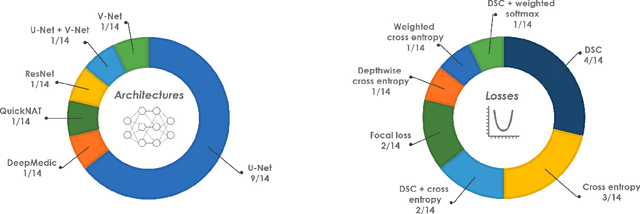
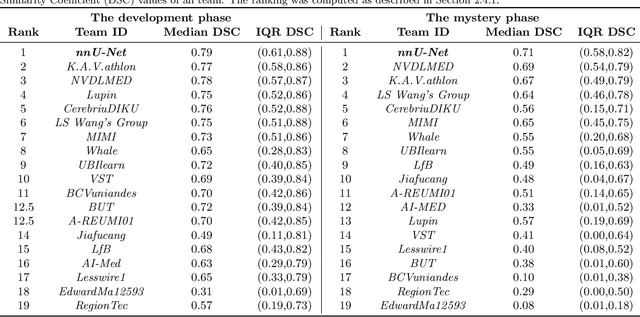
Abstract:International challenges have become the de facto standard for comparative assessment of image analysis algorithms given a specific task. Segmentation is so far the most widely investigated medical image processing task, but the various segmentation challenges have typically been organized in isolation, such that algorithm development was driven by the need to tackle a single specific clinical problem. We hypothesized that a method capable of performing well on multiple tasks will generalize well to a previously unseen task and potentially outperform a custom-designed solution. To investigate the hypothesis, we organized the Medical Segmentation Decathlon (MSD) - a biomedical image analysis challenge, in which algorithms compete in a multitude of both tasks and modalities. The underlying data set was designed to explore the axis of difficulties typically encountered when dealing with medical images, such as small data sets, unbalanced labels, multi-site data and small objects. The MSD challenge confirmed that algorithms with a consistent good performance on a set of tasks preserved their good average performance on a different set of previously unseen tasks. Moreover, by monitoring the MSD winner for two years, we found that this algorithm continued generalizing well to a wide range of other clinical problems, further confirming our hypothesis. Three main conclusions can be drawn from this study: (1) state-of-the-art image segmentation algorithms are mature, accurate, and generalize well when retrained on unseen tasks; (2) consistent algorithmic performance across multiple tasks is a strong surrogate of algorithmic generalizability; (3) the training of accurate AI segmentation models is now commoditized to non AI experts.
GASNet: Weakly-supervised Framework for COVID-19 Lesion Segmentation
Oct 19, 2020



Abstract:Segmentation of infected areas in chest CT volumes is of great significance for further diagnosis and treatment of COVID-19 patients. Due to the complex shapes and varied appearances of lesions, a large number of voxel-level labeled samples are generally required to train a lesion segmentation network, which is a main bottleneck for developing deep learning based medical image segmentation algorithms. In this paper, we propose a weakly-supervised lesion segmentation framework by embedding the Generative Adversarial training process into the Segmentation Network, which is called GASNet. GASNet is optimized to segment the lesion areas of a COVID-19 CT by the segmenter, and to replace the abnormal appearance with a generated normal appearance by the generator, so that the restored CT volumes are indistinguishable from healthy CT volumes by the discriminator. GASNet is supervised by chest CT volumes of many healthy and COVID-19 subjects without voxel-level annotations. Experiments on three public databases show that when using as few as one voxel-level labeled sample, the performance of GASNet is comparable to fully-supervised segmentation algorithms trained on dozens of voxel-level labeled samples.
CFUN: Combining Faster R-CNN and U-net Network for Efficient Whole Heart Segmentation
Dec 12, 2018



Abstract:In this paper, we propose a novel heart segmentation pipeline Combining Faster R-CNN and U-net Network (CFUN). Due to Faster R-CNN's precise localization ability and U-net's powerful segmentation ability, CFUN needs only one-step detection and segmentation inference to get the whole heart segmentation result, obtaining good results with significantly reduced computational cost. Besides, CFUN adopts a new loss function based on edge information named 3D Edge-loss as an auxiliary loss to accelerate the convergence of training and improve the segmentation results. Extensive experiments on the public dataset show that CFUN exhibits competitive segmentation performance in a sharply reduced inference time. Our source code and the model are publicly available at https://github.com/Wuziyi616/CFUN.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge