Yevgeniy Men
DRStageNet: Deep Learning for Diabetic Retinopathy Staging from Fundus Images
Dec 22, 2023



Abstract:Diabetic retinopathy (DR) is a prevalent complication of diabetes associated with a significant risk of vision loss. Timely identification is critical to curb vision impairment. Algorithms for DR staging from digital fundus images (DFIs) have been recently proposed. However, models often fail to generalize due to distribution shifts between the source domain on which the model was trained and the target domain where it is deployed. A common and particularly challenging shift is often encountered when the source- and target-domain supports do not fully overlap. In this research, we introduce DRStageNet, a deep learning model designed to mitigate this challenge. We used seven publicly available datasets, comprising a total of 93,534 DFIs that cover a variety of patient demographics, ethnicities, geographic origins and comorbidities. We fine-tune DINOv2, a pretrained model of self-supervised vision transformer, and implement a multi-source domain fine-tuning strategy to enhance generalization performance. We benchmark and demonstrate the superiority of our method to two state-of-the-art benchmarks, including a recently published foundation model. We adapted the grad-rollout method to our regression task in order to provide high-resolution explainability heatmaps. The error analysis showed that 59\% of the main errors had incorrect reference labels. DRStageNet is accessible at URL [upon acceptance of the manuscript].
Twice Regularized Markov Decision Processes: The Equivalence between Robustness and Regularization
Mar 12, 2023
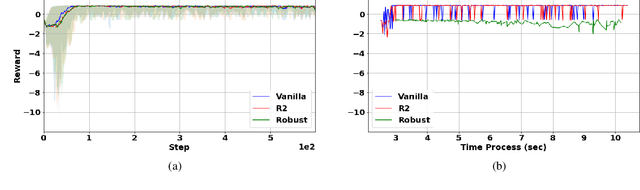
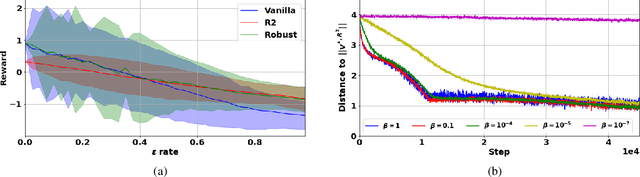
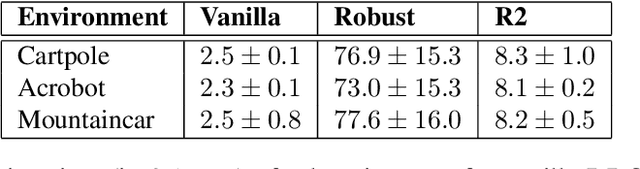
Abstract:Robust Markov decision processes (MDPs) aim to handle changing or partially known system dynamics. To solve them, one typically resorts to robust optimization methods. However, this significantly increases computational complexity and limits scalability in both learning and planning. On the other hand, regularized MDPs show more stability in policy learning without impairing time complexity. Yet, they generally do not encompass uncertainty in the model dynamics. In this work, we aim to learn robust MDPs using regularization. We first show that regularized MDPs are a particular instance of robust MDPs with uncertain reward. We thus establish that policy iteration on reward-robust MDPs can have the same time complexity as on regularized MDPs. We further extend this relationship to MDPs with uncertain transitions: this leads to a regularization term with an additional dependence on the value function. We then generalize regularized MDPs to twice regularized MDPs ($\text{R}^2$ MDPs), i.e., MDPs with $\textit{both}$ value and policy regularization. The corresponding Bellman operators enable us to derive planning and learning schemes with convergence and generalization guarantees, thus reducing robustness to regularization. We numerically show this two-fold advantage on tabular and physical domains, highlighting the fact that $\text{R}^2$ preserves its efficacy in continuous environments.
PVBM: A Python Vasculature Biomarker Toolbox Based On Retinal Blood Vessel Segmentation
Jul 31, 2022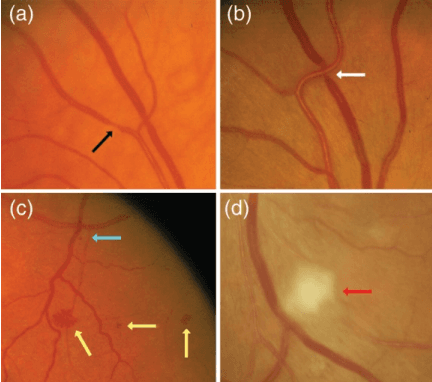
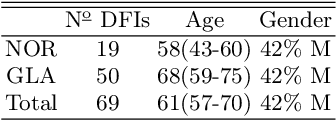
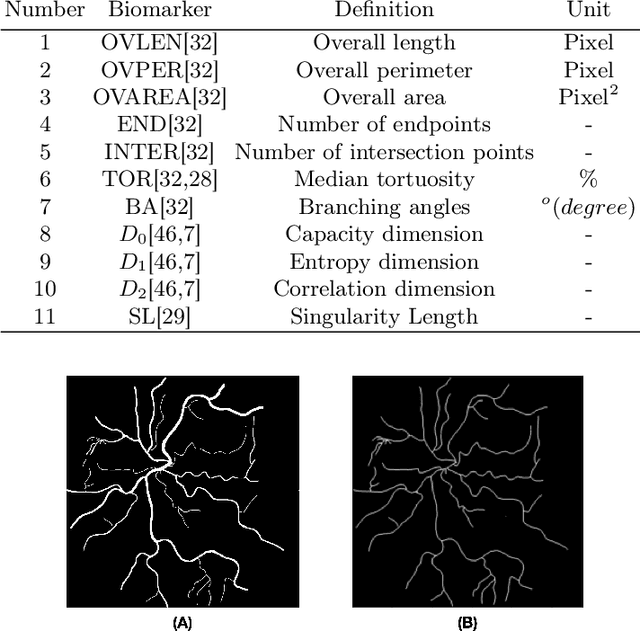
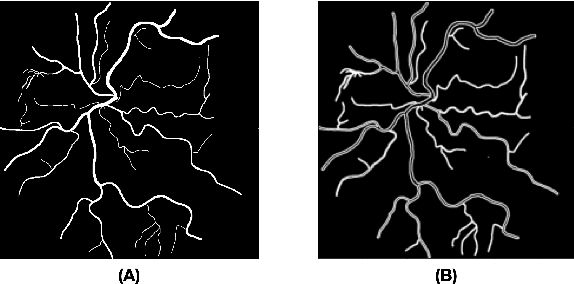
Abstract:Introduction: Blood vessels can be non-invasively visualized from a digital fundus image (DFI). Several studies have shown an association between cardiovascular risk and vascular features obtained from DFI. Recent advances in computer vision and image segmentation enable automatising DFI blood vessel segmentation. There is a need for a resource that can automatically compute digital vasculature biomarkers (VBM) from these segmented DFI. Methods: In this paper, we introduce a Python Vasculature BioMarker toolbox, denoted PVBM. A total of 11 VBMs were implemented. In particular, we introduce new algorithmic methods to estimate tortuosity and branching angles. Using PVBM, and as a proof of usability, we analyze geometric vascular differences between glaucomatous patients and healthy controls. Results: We built a fully automated vasculature biomarker toolbox based on DFI segmentations and provided a proof of usability to characterize the vascular changes in glaucoma. For arterioles and venules, all biomarkers were significant and lower in glaucoma patients compared to healthy controls except for tortuosity, venular singularity length and venular branching angles. Conclusion: We have automated the computation of 11 VBMs from retinal blood vessel segmentation. The PVBM toolbox is made open source under a GNU GPL 3 license and is available on physiozoo.com (following publication).
Generating Band-Limited Adversarial Surfaces Using Neural Networks
Nov 14, 2021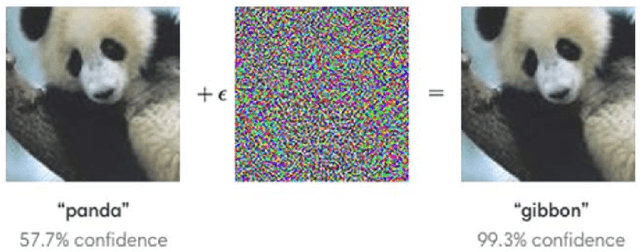
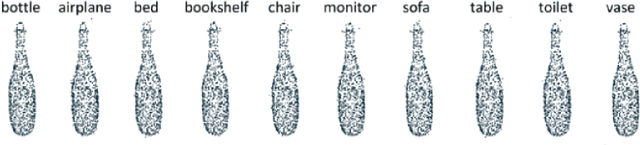
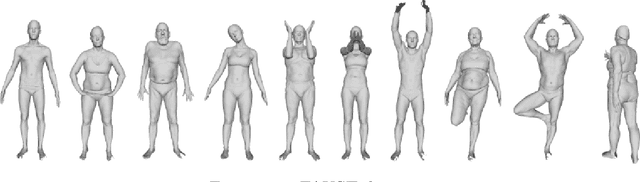
Abstract:Generating adversarial examples is the art of creating a noise that is added to an input signal of a classifying neural network, and thus changing the network's classification, while keeping the noise as tenuous as possible. While the subject is well-researched in the 2D regime, it is lagging behind in the 3D regime, i.e. attacking a classifying network that works on 3D point-clouds or meshes and, for example, classifies the pose of people's 3D scans. As of now, the vast majority of papers that describe adversarial attacks in this regime work by methods of optimization. In this technical report we suggest a neural network that generates the attacks. This network utilizes PointNet's architecture with some alterations. While the previous articles on which we based our work on have to optimize each shape separately, i.e. tailor an attack from scratch for each individual input without any learning, we attempt to create a unified model that can deduce the needed adversarial example with a single forward run.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge