Vilmundur Gudnason
Hip Fracture Prediction using the First Principal Component Derived from FEA-Computed Fracture Loads
Oct 03, 2022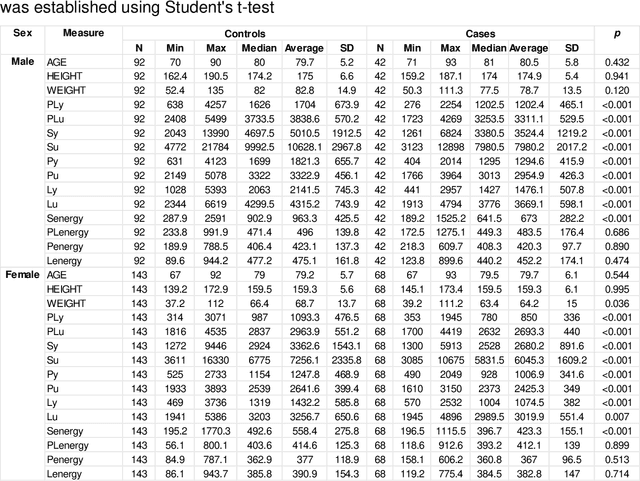
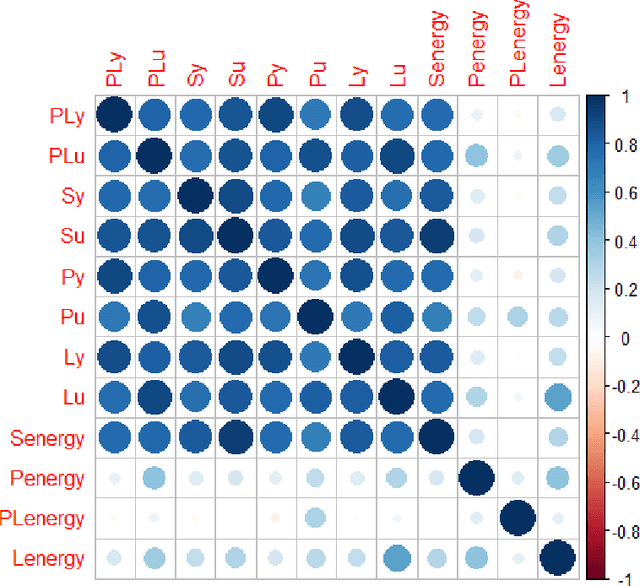
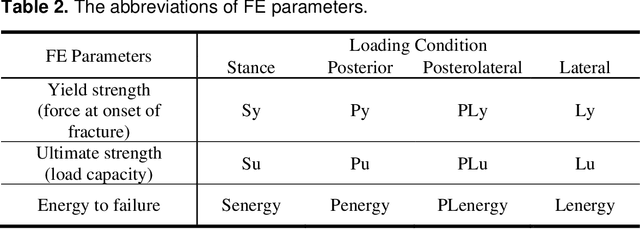
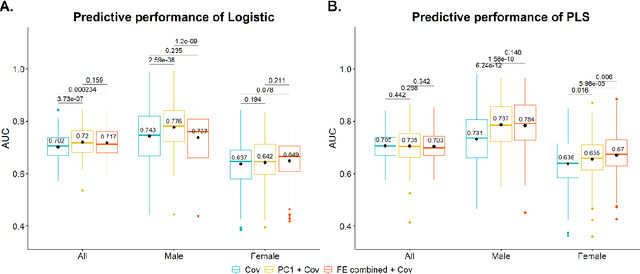
Abstract:Hip fracture risk assessment is an important but challenging task. Quantitative CT-based patient specific finite element analysis (FEA) computes the force (fracture load) to break the proximal femur in a particular loading condition. It provides different structural information about the proximal femur that can influence a subject overall fracture risk. To obtain a more robust measure of fracture risk, we used principal component analysis (PCA) to develop a global FEA computed fracture risk index that incorporates the FEA-computed yield and ultimate failure loads and energies to failure in four loading conditions (single-limb stance and impact from a fall onto the posterior, posterolateral, and lateral aspects of the greater trochanter) of 110 hip fracture subjects and 235 age and sex matched control subjects from the AGES-Reykjavik study. We found that the first PC (PC1) of the FE parameters was the only significant predictor of hip fracture. Using a logistic regression model, we determined if prediction performance for hip fracture using PC1 differed from that using FE parameters combined by stratified random resampling with respect to hip fracture status. The results showed that the average of the area under the receive operating characteristic curve (AUC) using PC1 was always higher than that using all FE parameters combined in the male subjects. The AUC of PC1 and AUC of the FE parameters combined were not significantly different than that in the female subjects or in all subjects
Fast and Robust Femur Segmentation from Computed Tomography Images for Patient-Specific Hip Fracture Risk Screening
Apr 20, 2022



Abstract:Osteoporosis is a common bone disease that increases the risk of bone fracture. Hip-fracture risk screening methods based on finite element analysis depend on segmented computed tomography (CT) images; however, current femur segmentation methods require manual delineations of large data sets. Here we propose a deep neural network for fully automated, accurate, and fast segmentation of the proximal femur from CT. Evaluation on a set of 1147 proximal femurs with ground truth segmentations demonstrates that our method is apt for hip-fracture risk screening, bringing us one step closer to a clinically viable option for screening at-risk patients for hip-fracture susceptibility.
Unsupervised brain lesion segmentation from MRI using a convolutional autoencoder
Nov 23, 2018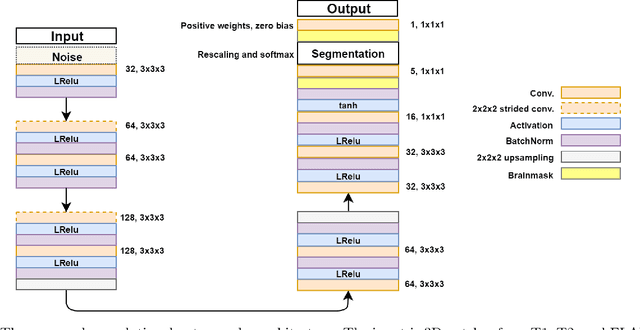
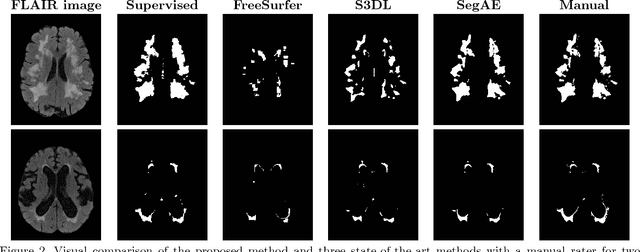

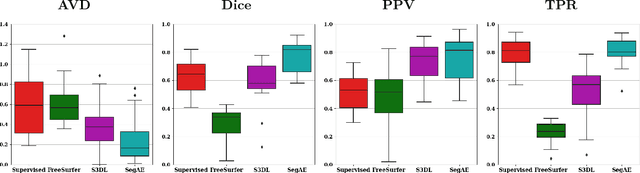
Abstract:Lesions that appear hyperintense in both Fluid Attenuated Inversion Recovery (FLAIR) and T2-weighted magnetic resonance images (MRIs) of the human brain are common in the brains of the elderly population and may be caused by ischemia or demyelination. Lesions are biomarkers for various neurodegenerative diseases, making accurate quantification of them important for both disease diagnosis and progression. Automatic lesion detection using supervised learning requires manually annotated images, which can often be impractical to acquire. Unsupervised lesion detection, on the other hand, does not require any manual delineation; however, these methods can be challenging to construct due to the variability in lesion load, placement of lesions, and voxel intensities. Here we present a novel approach to address this problem using a convolutional autoencoder, which learns to segment brain lesions as well as the white matter, gray matter, and cerebrospinal fluid by reconstructing FLAIR images as conical combinations of softmax layer outputs generated from the corresponding T1, T2, and FLAIR images. Some of the advantages of this model are that it accurately learns to segment lesions regardless of lesion load, and it can be used to quickly and robustly segment new images that were not in the training set. Comparisons with state-of-the-art segmentation methods evaluated on ground truth manual labels indicate that the proposed method works well for generating accurate lesion segmentations without the need for manual annotations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge