Vijay Pawar
Automated Detection of Acute Promyelocytic Leukemia in Blood Films and Bone Marrow Aspirates with Annotation-free Deep Learning
Mar 20, 2022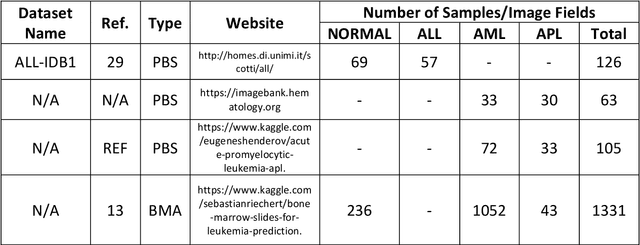
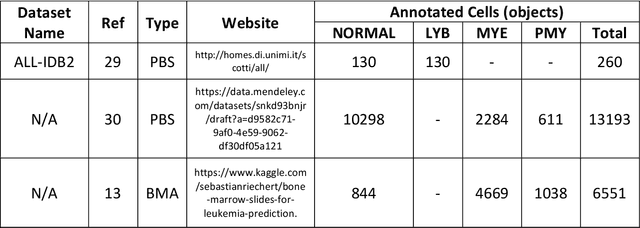
Abstract:While optical microscopy inspection of blood films and bone marrow aspirates by a hematologist is a crucial step in establishing diagnosis of acute leukemia, especially in low-resource settings where other diagnostic modalities might not be available, the task remains time-consuming and prone to human inconsistencies. This has an impact especially in cases of Acute Promyelocytic Leukemia (APL) that require urgent treatment. Integration of automated computational hematopathology into clinical workflows can improve the throughput of these services and reduce cognitive human error. However, a major bottleneck in deploying such systems is a lack of sufficient cell morphological object-labels annotations to train deep learning models. We overcome this by leveraging patient diagnostic labels to train weakly-supervised models that detect different types of acute leukemia. We introduce a deep learning approach, Multiple Instance Learning for Leukocyte Identification (MILLIE), able to perform automated reliable analysis of blood films with minimal supervision. Without being trained to classify individual cells, MILLIE differentiates between acute lymphoblastic and myeloblastic leukemia in blood films. More importantly, MILLIE detects APL in blood films (AUC 0.94+/-0.04) and in bone marrow aspirates (AUC 0.99+/-0.01). MILLIE is a viable solution to augment the throughput of clinical pathways that require assessment of blood film microscopy.
Autonomous object harvesting using synchronized optoelectronic microrobots
Mar 08, 2021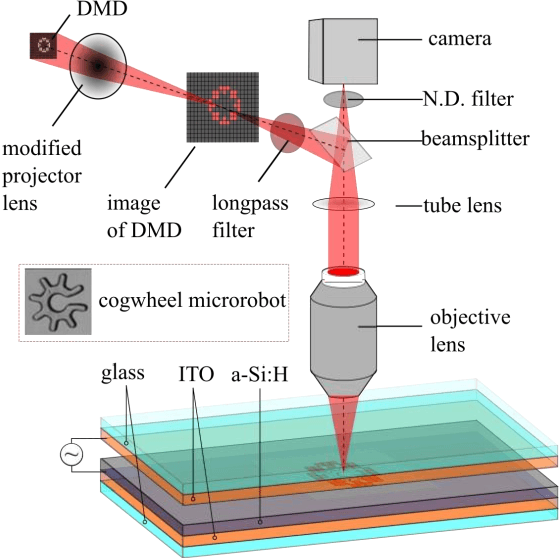
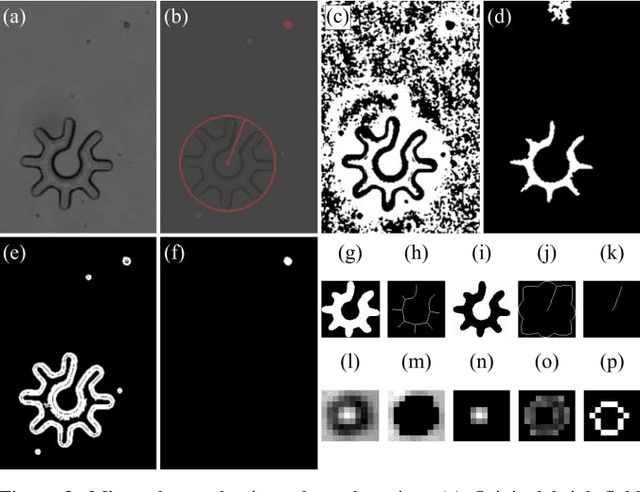
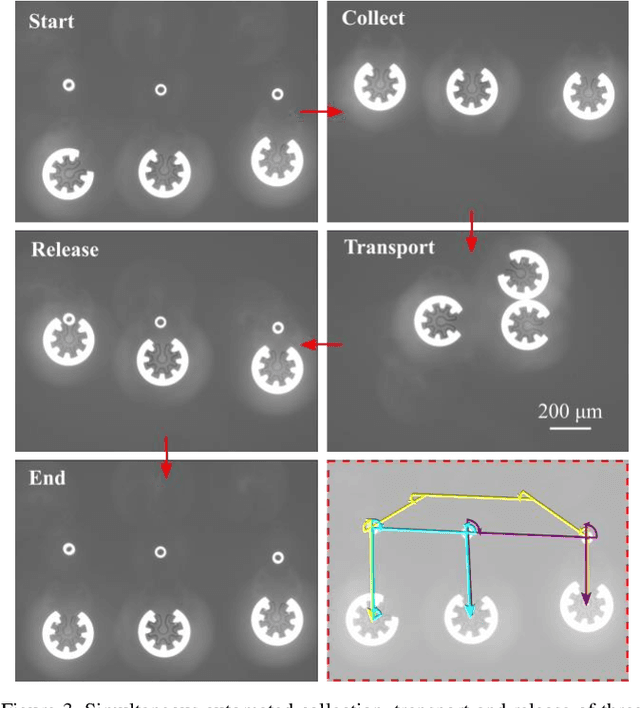
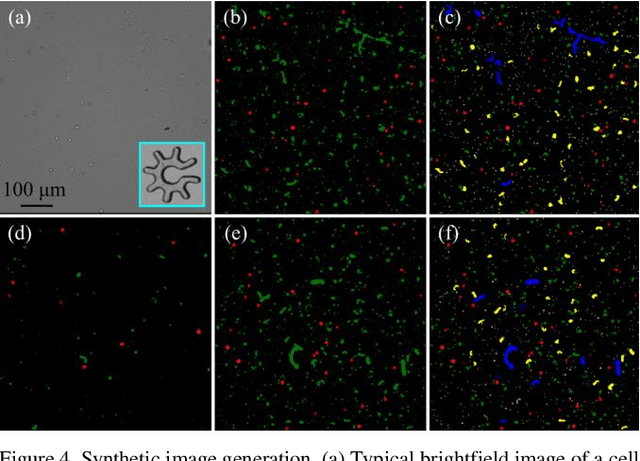
Abstract:Optoelectronic tweezer-driven microrobots (OETdMs) are a versatile micromanipulation technology based on the use of light induced dielectrophoresis to move small dielectric structures (microrobots) across a photoconductive substrate. The microrobots in turn can be used to exert forces on secondary objects and carry out a wide range of micromanipulation operations, including collecting, transporting and depositing microscopic cargos. In contrast to alternative (direct) micromanipulation techniques, OETdMs are relatively gentle, making them particularly well suited to interacting with sensitive objects such as biological cells. However, at present such systems are used exclusively under manual control by a human operator. This limits the capacity for simultaneous control of multiple microrobots, reducing both experimental throughput and the possibility of cooperative multi-robot operations. In this article, we describe an approach to automated targeting and path planning to enable open-loop control of multiple microrobots. We demonstrate the performance of the method in practice, using microrobots to simultaneously collect, transport and deposit silica microspheres. Using computational simulations based on real microscopic image data, we investigate the capacity of microrobots to collect target cells from within a dissociated tissue culture. Our results indicate the feasibility of using OETdMs to autonomously carry out micromanipulation tasks within complex, unstructured environments.
Whole-Sample Mapping of Cancerous and Benign Tissue Properties
Jul 23, 2019



Abstract:Structural and mechanical differences between cancerous and healthy tissue give rise to variations in macroscopic properties such as visual appearance and elastic modulus that show promise as signatures for early cancer detection. Atomic force microscopy (AFM) has been used to measure significant differences in stiffness between cancerous and healthy cells owing to its high force sensitivity and spatial resolution, however due to absorption and scattering of light, it is often challenging to accurately locate where AFM measurements have been made on a bulk tissue sample. In this paper we describe an image registration method that localizes AFM elastic stiffness measurements with high-resolution images of haematoxylin and eosin (H\&E)-stained tissue to within 1.5 microns. Color RGB images are segmented into three structure types (lumen, cells and stroma) by a neural network classifier trained on ground-truth pixel data obtained through k-means clustering in HSV color space. Using the localized stiffness maps and corresponding structural information, a whole-sample stiffness map is generated with a region matching and interpolation algorithm that associates similar structures with measured stiffness values. We present results showing significant differences in stiffness between healthy and cancerous liver tissue and discuss potential applications of this technique.
Data-Driven Malaria Prevalence Prediction in Large Densely-Populated Urban Holoendemic sub-Saharan West Africa: Harnessing Machine Learning Approaches and 22-years of Prospectively Collected Data
Jun 18, 2019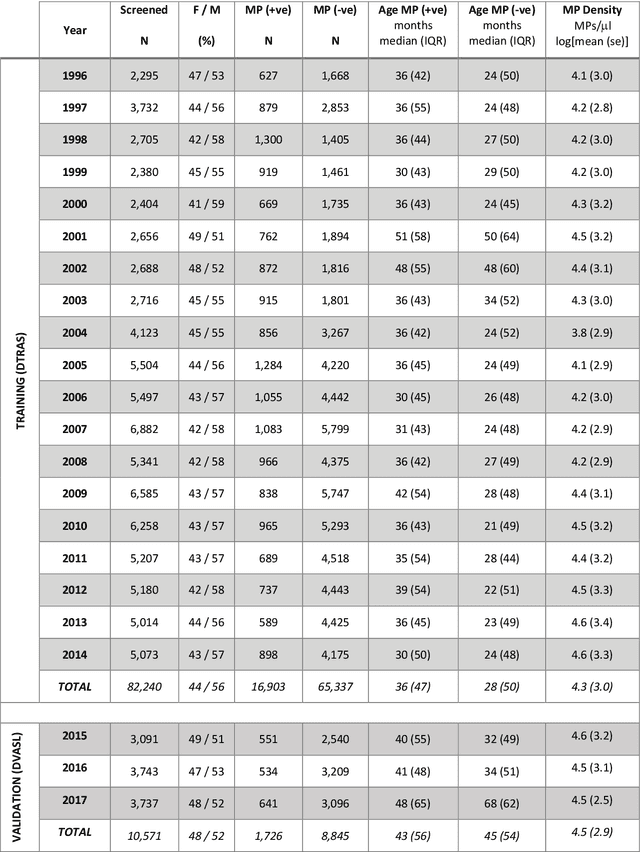
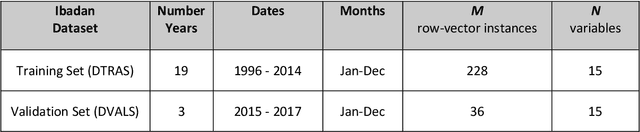
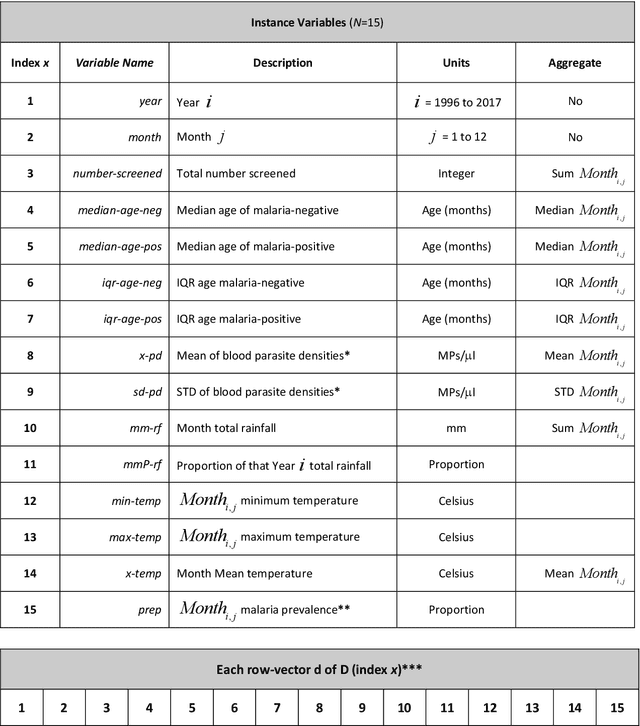
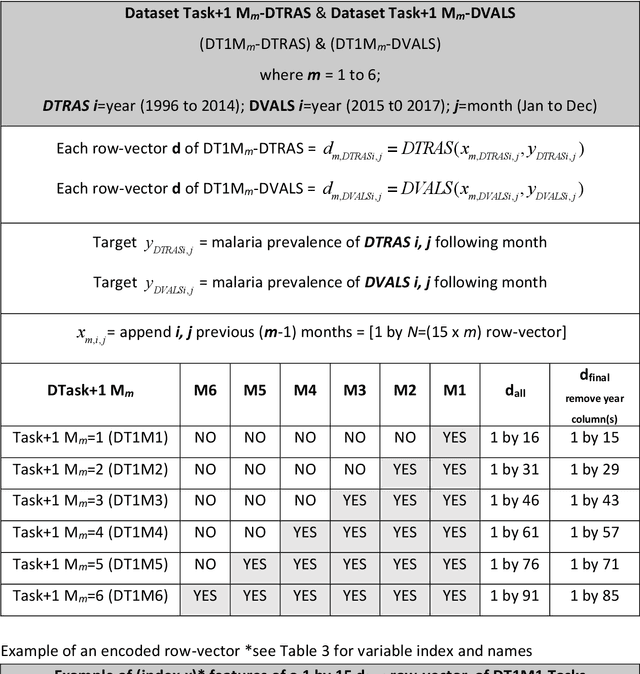
Abstract:Plasmodium falciparum malaria still poses one of the greatest threats to human life with over 200 million cases globally leading to half-million deaths annually. Of these, 90% of cases and of the mortality occurs in sub-Saharan Africa, mostly among children. Although malaria prediction systems are central to the 2016-2030 malaria Global Technical Strategy, currently these are inadequate at capturing and estimating the burden of disease in highly endemic countries. We developed and validated a computational system that exploits the predictive power of current Machine Learning approaches on 22-years of prospective data from the high-transmission holoendemic malaria urban-densely-populated sub-Saharan West-Africa metropolis of Ibadan. Our dataset of >9x104 screened study participants attending our clinical and community services from 1996 to 2017 contains monthly prevalence, temporal, environmental and host features. Our Locality-specific Elastic-Net based Malaria Prediction System (LEMPS) achieves good generalization performance, both in magnitude and direction of the prediction, when tasked to predict monthly prevalence on previously unseen validation data (MAE<=6x10-2, MSE<=7x10-3) within a range of (+0.1 to -0.05) error-tolerance which is relevant and usable for aiding decision-support in a holoendemic setting. LEMPS is well-suited for malaria prediction, where there are multiple features which are correlated with one another, and trading-off between regularization-strength L1-norm and L2-norm allows the system to retain stability. Data-driven systems are critical for regionally-adaptable surveillance, management of control strategies and resource allocation across stretched healthcare systems.
Deep Learning Enhanced Extended Depth-of-Field for Thick Blood-Film Malaria High-Throughput Microscopy
Jun 18, 2019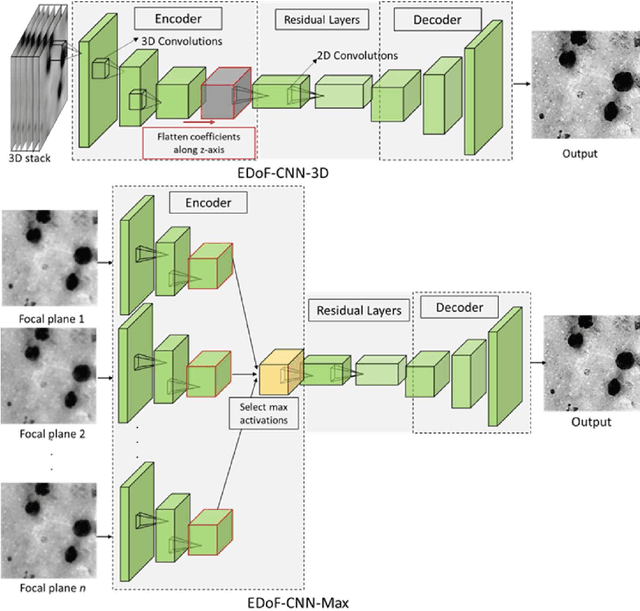
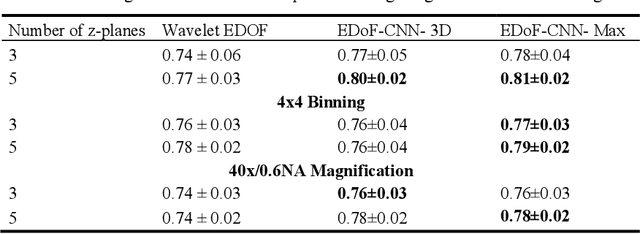
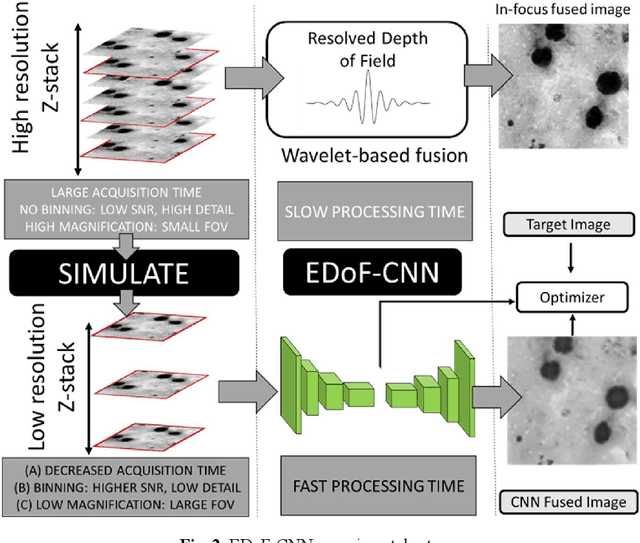
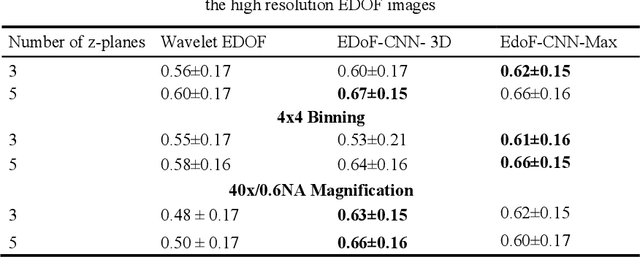
Abstract:Fast accurate diagnosis of malaria is still a global health challenge for which automated digital-pathology approaches could provide scalable solutions amenable to be deployed in low-to-middle income countries. Here we address the problem of Extended Depth-of-Field (EDoF) in thick blood film microscopy for rapid automated malaria diagnosis. High magnification oil-objectives (100x) with large numerical aperture are usually preferred to resolve the fine structural details that help separate true parasites from distractors. However, such objectives have a very limited depth-of-field requiring the acquisition of a series of images at different focal planes per field of view (FOV). Current EDoF techniques based on multi-scale decompositions are time consuming and therefore not suited for high-throughput analysis of specimens. To overcome this challenge, we developed a new deep learning method based on Convolutional Neural Networks (EDoF-CNN) that is able to rapidly perform the extended depth-of-field while also enhancing the spatial resolution of the resulting fused image. We evaluated our approach using simulated low-resolution z-stacks from Giemsa-stained thick blood smears from patients presenting with Plasmodium falciparum malaria. The EDoF-CNN allows speed-up of our digital-pathology acquisition platform and significantly improves the quality of the EDoF compared to the traditional multi-scaled approaches when applied to lower resolution stacks corresponding to acquisitions with fewer focal planes, large camera pixel binning or lower magnification objectives (larger FOV). We use the parasite detection accuracy of a deep learning model on the EDoFs as a concrete, task-specific measure of performance of this approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge