Christopher Bendkowski
Automated Detection of Acute Promyelocytic Leukemia in Blood Films and Bone Marrow Aspirates with Annotation-free Deep Learning
Mar 20, 2022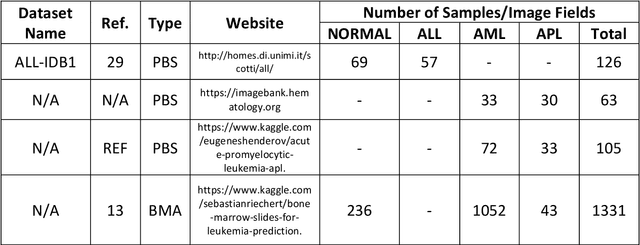
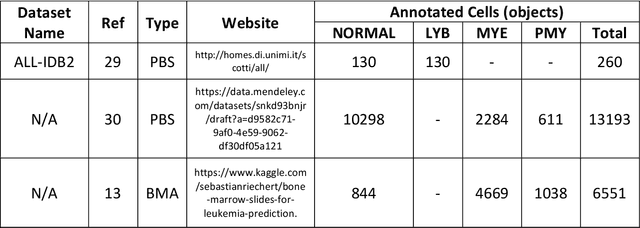
Abstract:While optical microscopy inspection of blood films and bone marrow aspirates by a hematologist is a crucial step in establishing diagnosis of acute leukemia, especially in low-resource settings where other diagnostic modalities might not be available, the task remains time-consuming and prone to human inconsistencies. This has an impact especially in cases of Acute Promyelocytic Leukemia (APL) that require urgent treatment. Integration of automated computational hematopathology into clinical workflows can improve the throughput of these services and reduce cognitive human error. However, a major bottleneck in deploying such systems is a lack of sufficient cell morphological object-labels annotations to train deep learning models. We overcome this by leveraging patient diagnostic labels to train weakly-supervised models that detect different types of acute leukemia. We introduce a deep learning approach, Multiple Instance Learning for Leukocyte Identification (MILLIE), able to perform automated reliable analysis of blood films with minimal supervision. Without being trained to classify individual cells, MILLIE differentiates between acute lymphoblastic and myeloblastic leukemia in blood films. More importantly, MILLIE detects APL in blood films (AUC 0.94+/-0.04) and in bone marrow aspirates (AUC 0.99+/-0.01). MILLIE is a viable solution to augment the throughput of clinical pathways that require assessment of blood film microscopy.
Autonomous object harvesting using synchronized optoelectronic microrobots
Mar 08, 2021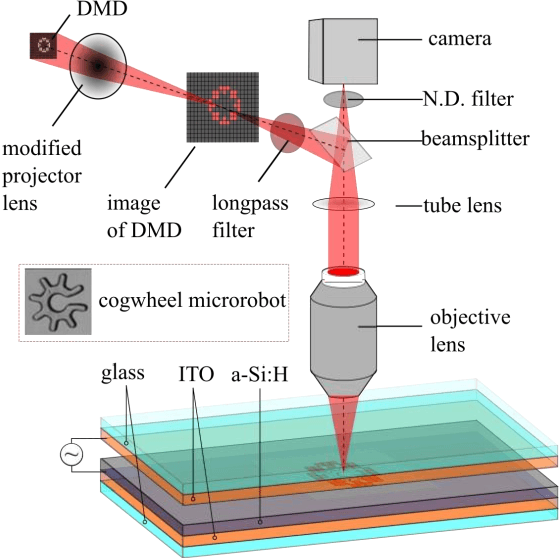
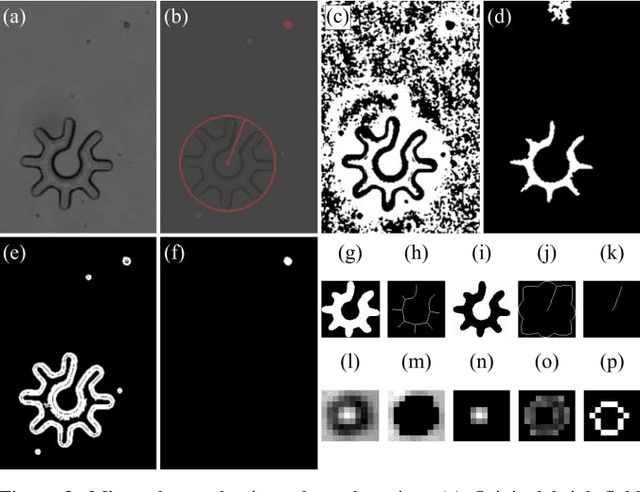
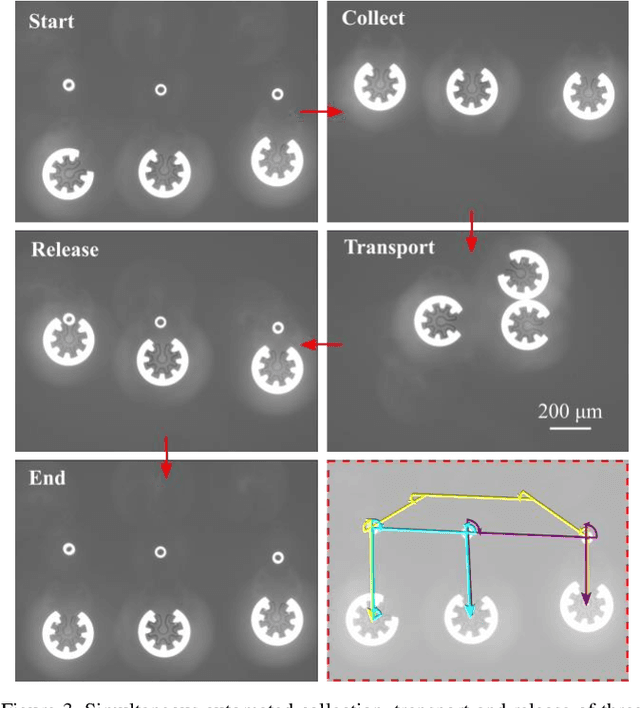
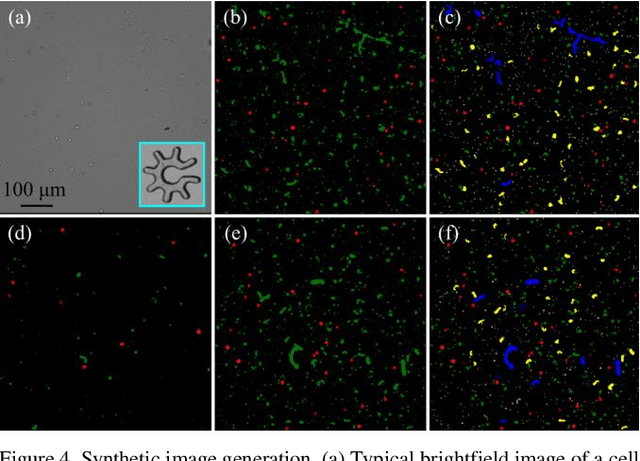
Abstract:Optoelectronic tweezer-driven microrobots (OETdMs) are a versatile micromanipulation technology based on the use of light induced dielectrophoresis to move small dielectric structures (microrobots) across a photoconductive substrate. The microrobots in turn can be used to exert forces on secondary objects and carry out a wide range of micromanipulation operations, including collecting, transporting and depositing microscopic cargos. In contrast to alternative (direct) micromanipulation techniques, OETdMs are relatively gentle, making them particularly well suited to interacting with sensitive objects such as biological cells. However, at present such systems are used exclusively under manual control by a human operator. This limits the capacity for simultaneous control of multiple microrobots, reducing both experimental throughput and the possibility of cooperative multi-robot operations. In this article, we describe an approach to automated targeting and path planning to enable open-loop control of multiple microrobots. We demonstrate the performance of the method in practice, using microrobots to simultaneously collect, transport and deposit silica microspheres. Using computational simulations based on real microscopic image data, we investigate the capacity of microrobots to collect target cells from within a dissociated tissue culture. Our results indicate the feasibility of using OETdMs to autonomously carry out micromanipulation tasks within complex, unstructured environments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge