Song He
Bell Laboratories, Lucent Technologies, Murray Hill, NJ
Towards Cross-Modal Text-Molecule Retrieval with Better Modality Alignment
Oct 31, 2024Abstract:Cross-modal text-molecule retrieval model aims to learn a shared feature space of the text and molecule modalities for accurate similarity calculation, which facilitates the rapid screening of molecules with specific properties and activities in drug design. However, previous works have two main defects. First, they are inadequate in capturing modality-shared features considering the significant gap between text sequences and molecule graphs. Second, they mainly rely on contrastive learning and adversarial training for cross-modality alignment, both of which mainly focus on the first-order similarity, ignoring the second-order similarity that can capture more structural information in the embedding space. To address these issues, we propose a novel cross-modal text-molecule retrieval model with two-fold improvements. Specifically, on the top of two modality-specific encoders, we stack a memory bank based feature projector that contain learnable memory vectors to extract modality-shared features better. More importantly, during the model training, we calculate four kinds of similarity distributions (text-to-text, text-to-molecule, molecule-to-molecule, and molecule-to-text similarity distributions) for each instance, and then minimize the distance between these similarity distributions (namely second-order similarity losses) to enhance cross-modal alignment. Experimental results and analysis strongly demonstrate the effectiveness of our model. Particularly, our model achieves SOTA performance, outperforming the previously-reported best result by 6.4%.
Domain-Adversarial Multi-Task Framework for Novel Therapeutic Property Prediction of Compounds
Sep 28, 2018
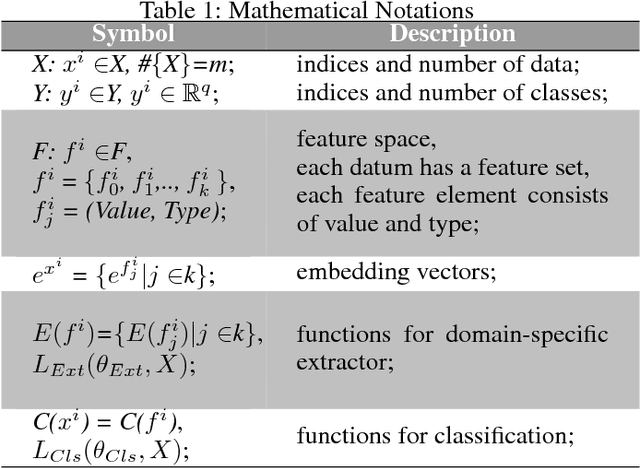
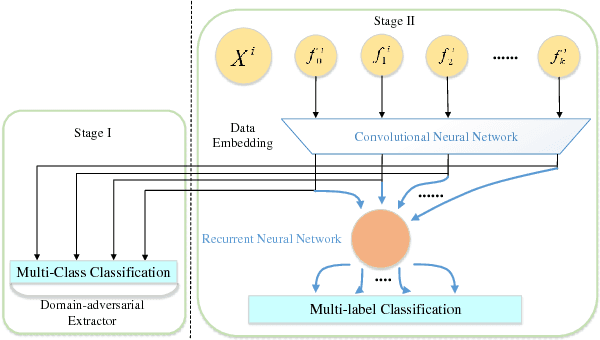
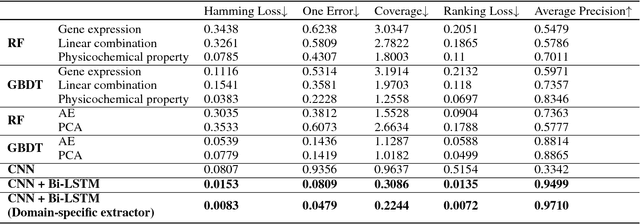
Abstract:With the rapid development of high-throughput technologies, parallel acquisition of large-scale drug-informatics data provides huge opportunities to improve pharmaceutical research and development. One significant application is the purpose prediction of small molecule compounds, aiming to specify therapeutic properties of extensive purpose-unknown compounds and to repurpose novel therapeutic properties of FDA-approved drugs. Such problem is very challenging since compound attributes contain heterogeneous data with various feature patterns such as drug fingerprint, drug physicochemical property, drug perturbation gene expression. Moreover, there is complex nonlinear dependency among heterogeneous data. In this paper, we propose a novel domain-adversarial multi-task framework for integrating shared knowledge from multiple domains. The framework utilizes the adversarial strategy to effectively learn target representations and models their nonlinear dependency. Experiments on two real-world datasets illustrate that the performance of our approach obtains an obvious improvement over competitive baselines. The novel therapeutic properties of purpose-unknown compounds we predicted are mostly reported or brought to the clinics. Furthermore, our framework can integrate various attributes beyond the three domains examined here and can be applied in the industry for screening the purpose of huge amounts of as yet unidentified compounds. Source codes of this paper are available on Github.
Stabilizing the Richardson Algorithm by Controlling Chaos
Jun 11, 1996Abstract:By viewing the operations of the Richardson purification algorithm as a discrete time dynamical process, we propose a method to overcome the instability of the algorithm by controlling chaos. We present theoretical analysis and numerical results on the behavior and performance of the stabilized algorithm.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge