Simon Pezold
Standardized Assessment of Automatic Segmentation of White Matter Hyperintensities and Results of the WMH Segmentation Challenge
Apr 01, 2019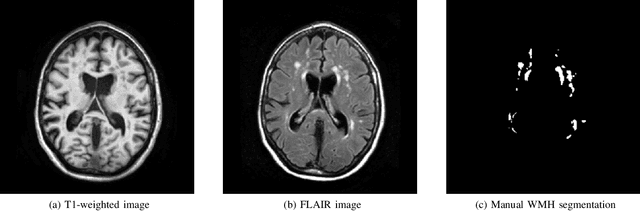
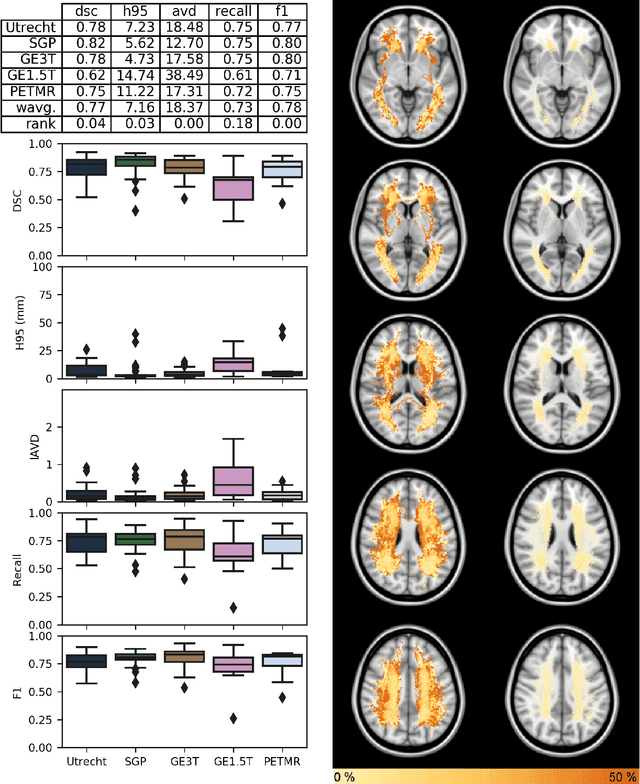
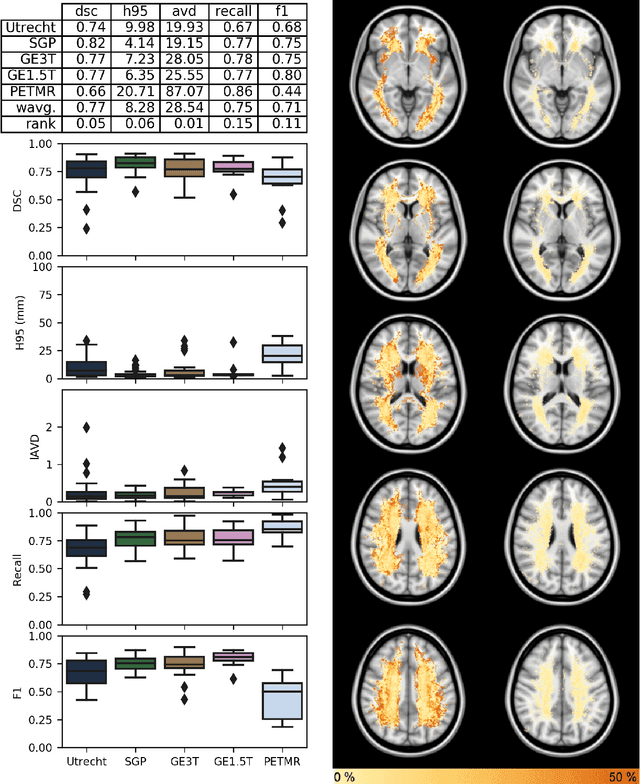
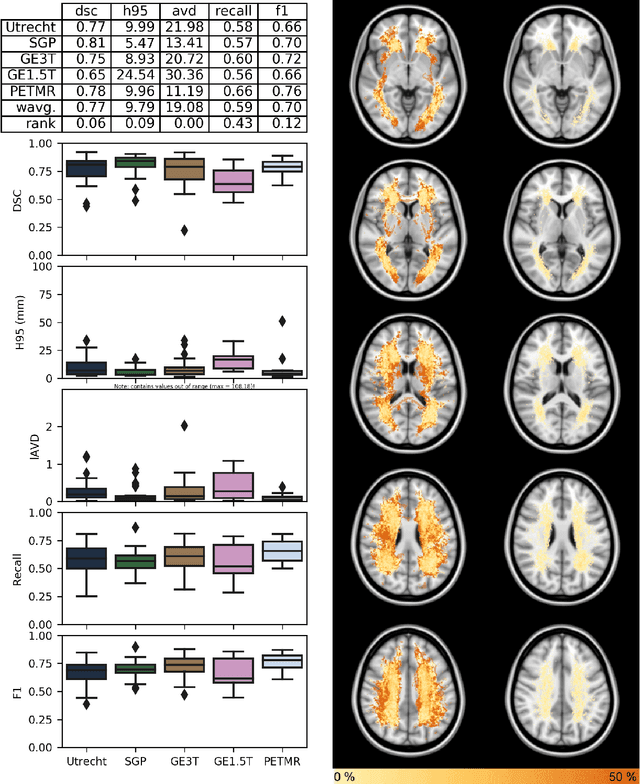
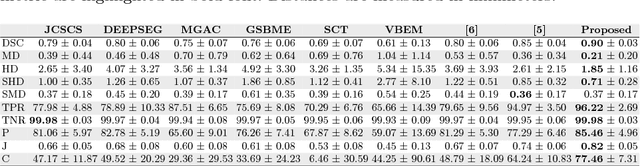
Abstract:Quantification of cerebral white matter hyperintensities (WMH) of presumed vascular origin is of key importance in many neurological research studies. Currently, measurements are often still obtained from manual segmentations on brain MR images, which is a laborious procedure. Automatic WMH segmentation methods exist, but a standardized comparison of the performance of such methods is lacking. We organized a scientific challenge, in which developers could evaluate their method on a standardized multi-center/-scanner image dataset, giving an objective comparison: the WMH Segmentation Challenge (https://wmh.isi.uu.nl/). Sixty T1+FLAIR images from three MR scanners were released with manual WMH segmentations for training. A test set of 110 images from five MR scanners was used for evaluation. Segmentation methods had to be containerized and submitted to the challenge organizers. Five evaluation metrics were used to rank the methods: (1) Dice similarity coefficient, (2) modified Hausdorff distance (95th percentile), (3) absolute log-transformed volume difference, (4) sensitivity for detecting individual lesions, and (5) F1-score for individual lesions. Additionally, methods were ranked on their inter-scanner robustness. Twenty participants submitted their method for evaluation. This paper provides a detailed analysis of the results. In brief, there is a cluster of four methods that rank significantly better than the other methods, with one clear winner. The inter-scanner robustness ranking shows that not all methods generalize to unseen scanners. The challenge remains open for future submissions and provides a public platform for method evaluation.
Spinal Cord Gray Matter-White Matter Segmentation on Magnetic Resonance AMIRA Images with MD-GRU
Aug 07, 2018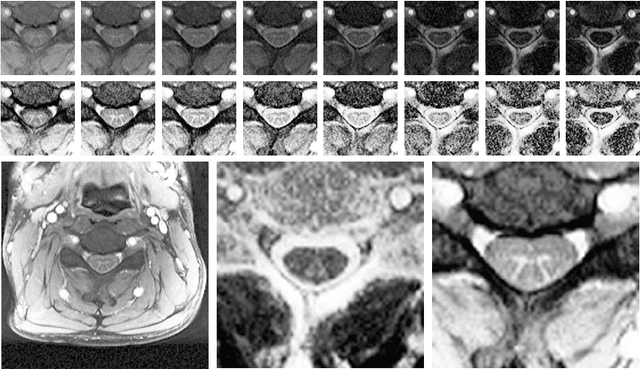
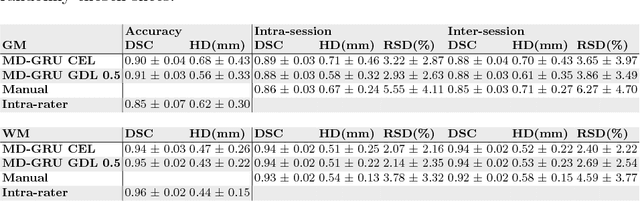
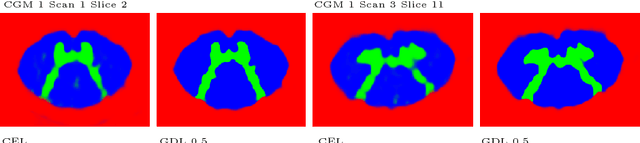
Abstract:The small butterfly shaped structure of spinal cord (SC) gray matter (GM) is challenging to image and to delinate from its surrounding white matter (WM). Segmenting GM is up to a point a trade-off between accuracy and precision. We propose a new pipeline for GM-WM magnetic resonance (MR) image acquisition and segmentation. We report superior results as compared to the ones recently reported in the SC GM segmentation challenge and show even better results using the averaged magnetization inversion recovery acquisitions (AMIRA) sequence. Scan-rescan experiments with the AMIRA sequence show high reproducibility in terms of Dice coefficient, Hausdorff distance and relative standard deviation. We use a recurrent neural network (RNN) with multi-dimensional gated recurrent units (MD-GRU) to train segmentation models on the AMIRA dataset of 855 slices. We added a generalized dice loss to the cross entropy loss that MD-GRU uses and were able to improve the results.
Pathology Segmentation using Distributional Differences to Images of Healthy Origin
May 25, 2018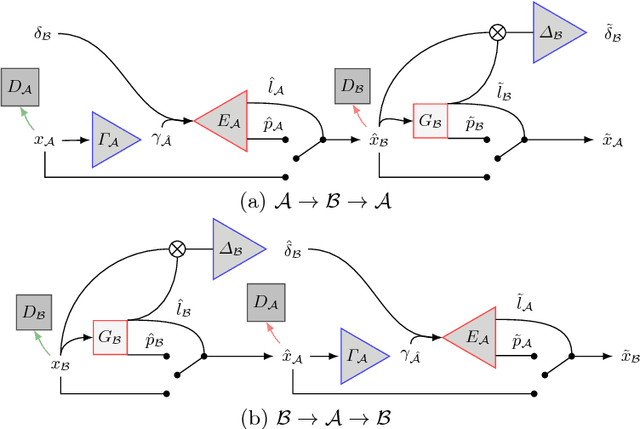
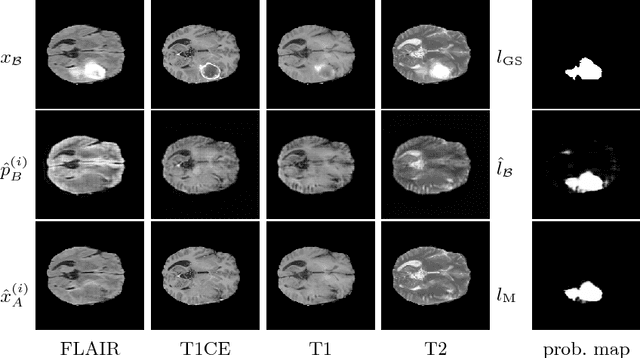
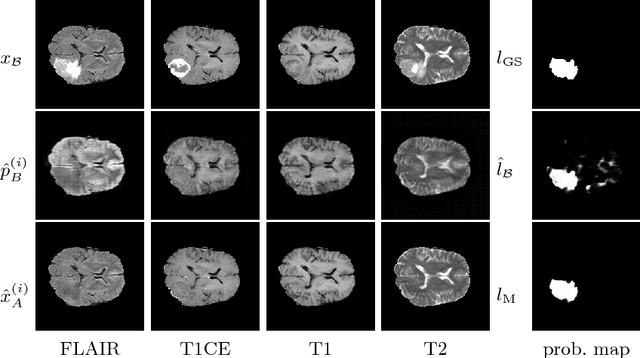
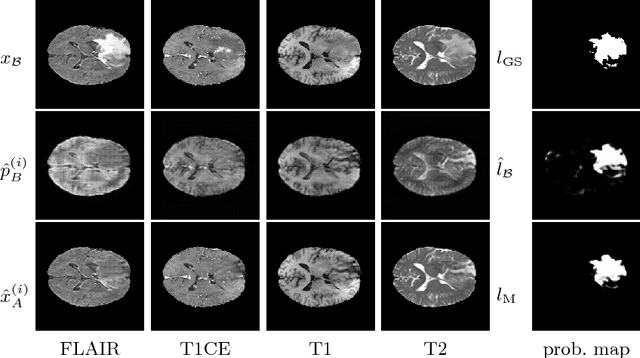
Abstract:We present a method to model pathologies in medical data, trained on data labelled on the image level as healthy or containing a visual defect. Our model not only allows us to create pixelwise semantic segmentations, it is also able to create inpaintings for the segmentations to render the pathological image healthy. Furthermore, we can draw new unseen pathology samples from this model based on the distribution in the data. We show quantitatively, that our method is able to segment pathologies with a surprising accuracy and show qualitative results of both the segmentations and inpaintings. A comparison with a supervised segmentation method indicates, that the accuracy of our proposed weakly-supervised segmentation is nevertheless quite close.
Multi-dimensional Gated Recurrent Units for Automated Anatomical Landmark Localization
Aug 09, 2017



Abstract:We present an automated method for localizing an anatomical landmark in three-dimensional medical images. The method combines two recurrent neural networks in a coarse-to-fine approach: The first network determines a candidate neighborhood by analyzing the complete given image volume. The second network localizes the actual landmark precisely and accurately in the candidate neighborhood. Both networks take advantage of multi-dimensional gated recurrent units in their main layers, which allow for high model complexity with a comparatively small set of parameters. We localize the medullopontine sulcus in 3D magnetic resonance images of the head and neck. We show that the proposed approach outperforms similar localization techniques both in terms of mean distance in millimeters and voxels w.r.t. manual labelings of the data. With a mean localization error of 1.7 mm, the proposed approach performs on par with neurological experts, as we demonstrate in an interrater comparison.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge