Simeng Zhu
Atlas is Your Perfect Context: One-Shot Customization for Generalizable Foundational Medical Image Segmentation
Dec 20, 2025



Abstract:Accurate medical image segmentation is essential for clinical diagnosis and treatment planning. While recent interactive foundation models (e.g., nnInteractive) enhance generalization through large-scale multimodal pretraining, they still depend on precise prompts and often perform below expectations in contexts that are underrepresented in their training data. We present AtlasSegFM, an atlas-guided framework that customizes available foundation models to clinical contexts with a single annotated example. The core innovations are: 1) a pipeline that provides context-aware prompts for foundation models via registration between a context atlas and query images, and 2) a test-time adapter to fuse predictions from both atlas registration and the foundation model. Extensive experiments across public and in-house datasets spanning multiple modalities and organs demonstrate that AtlasSegFM consistently improves segmentation, particularly for small, delicate structures. AtlasSegFM provides a lightweight, deployable solution one-shot customization of foundation models in real-world clinical workflows. The code will be made publicly available.
Modality-AGnostic Image Cascade (MAGIC) for Multi-Modality Cardiac Substructure Segmentation
Jun 12, 2025Abstract:Cardiac substructures are essential in thoracic radiation therapy planning to minimize risk of radiation-induced heart disease. Deep learning (DL) offers efficient methods to reduce contouring burden but lacks generalizability across different modalities and overlapping structures. This work introduces and validates a Modality-AGnostic Image Cascade (MAGIC) for comprehensive and multi-modal cardiac substructure segmentation. MAGIC is implemented through replicated encoding and decoding branches of an nnU-Net-based, U-shaped backbone conserving the function of a single model. Twenty cardiac substructures (heart, chambers, great vessels (GVs), valves, coronary arteries (CAs), and conduction nodes) from simulation CT (Sim-CT), low-field MR-Linac, and cardiac CT angiography (CCTA) modalities were manually delineated and used to train (n=76), validate (n=15), and test (n=30) MAGIC. Twelve comparison models (four segmentation subgroups across three modalities) were equivalently trained. All methods were compared for training efficiency and against reference contours using the Dice Similarity Coefficient (DSC) and two-tailed Wilcoxon Signed-Rank test (threshold, p<0.05). Average DSC scores were 0.75(0.16) for Sim-CT, 0.68(0.21) for MR-Linac, and 0.80(0.16) for CCTA. MAGIC outperforms the comparison in 57% of cases, with limited statistical differences. MAGIC offers an effective and accurate segmentation solution that is lightweight and capable of segmenting multiple modalities and overlapping structures in a single model. MAGIC further enables clinical implementation by simplifying the computational requirements and offering unparalleled flexibility for clinical settings.
Prostate Cancer Malignancy Detection and localization from mpMRI using auto-Deep Learning: One Step Closer to Clinical Utilization
Jun 13, 2022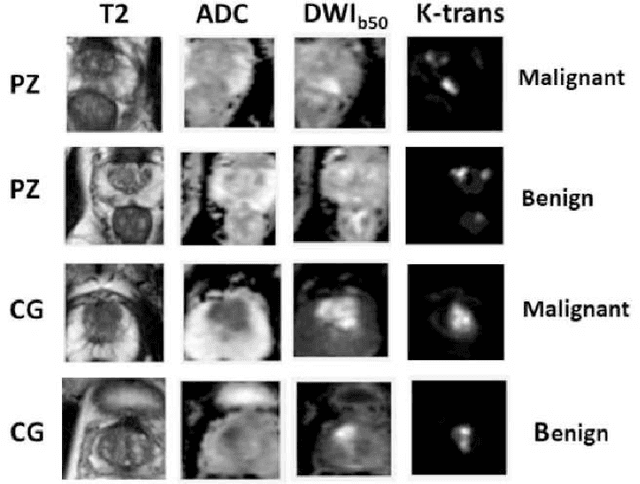

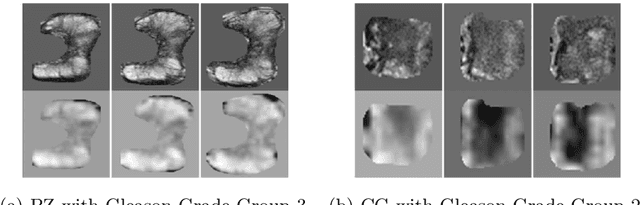
Abstract:Automatic diagnosis of malignant prostate cancer patients from mpMRI has been studied heavily in the past years. Model interpretation and domain drift have been the main road blocks for clinical utilization. As an extension from our previous work where we trained a customized convolutional neural network on a public cohort with 201 patients and the cropped 2D patches around the region of interest were used as the input, the cropped 2.5D slices of the prostate glands were used as the input, and the optimal model were searched in the model space using autoKeras. Something different was peripheral zone (PZ) and central gland (CG) were trained and tested separately, the PZ detector and CG detector were demonstrated effectively in highlighting the most suspicious slices out of a sequence, hopefully to greatly ease the workload for the physicians.
OpenKBP-Opt: An international and reproducible evaluation of 76 knowledge-based planning pipelines
Feb 16, 2022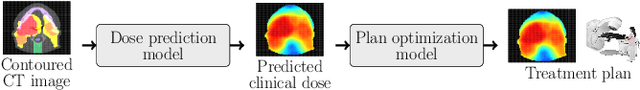

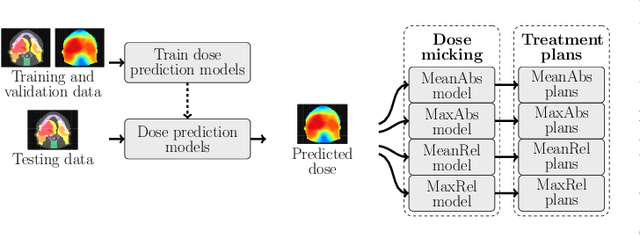
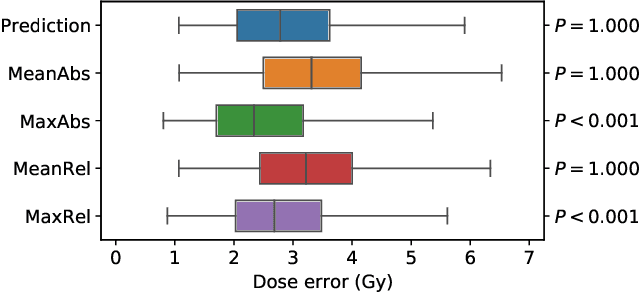
Abstract:We establish an open framework for developing plan optimization models for knowledge-based planning (KBP) in radiotherapy. Our framework includes reference plans for 100 patients with head-and-neck cancer and high-quality dose predictions from 19 KBP models that were developed by different research groups during the OpenKBP Grand Challenge. The dose predictions were input to four optimization models to form 76 unique KBP pipelines that generated 7600 plans. The predictions and plans were compared to the reference plans via: dose score, which is the average mean absolute voxel-by-voxel difference in dose a model achieved; the deviation in dose-volume histogram (DVH) criterion; and the frequency of clinical planning criteria satisfaction. We also performed a theoretical investigation to justify our dose mimicking models. The range in rank order correlation of the dose score between predictions and their KBP pipelines was 0.50 to 0.62, which indicates that the quality of the predictions is generally positively correlated with the quality of the plans. Additionally, compared to the input predictions, the KBP-generated plans performed significantly better (P<0.05; one-sided Wilcoxon test) on 18 of 23 DVH criteria. Similarly, each optimization model generated plans that satisfied a higher percentage of criteria than the reference plans. Lastly, our theoretical investigation demonstrated that the dose mimicking models generated plans that are also optimal for a conventional planning model. This was the largest international effort to date for evaluating the combination of KBP prediction and optimization models. In the interest of reproducibility, our data and code is freely available at https://github.com/ababier/open-kbp-opt.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge