Shameema Sikder
StepAL: Step-aware Active Learning for Cataract Surgical Videos
Jul 29, 2025Abstract:Active learning (AL) can reduce annotation costs in surgical video analysis while maintaining model performance. However, traditional AL methods, developed for images or short video clips, are suboptimal for surgical step recognition due to inter-step dependencies within long, untrimmed surgical videos. These methods typically select individual frames or clips for labeling, which is ineffective for surgical videos where annotators require the context of the entire video for annotation. To address this, we propose StepAL, an active learning framework designed for full video selection in surgical step recognition. StepAL integrates a step-aware feature representation, which leverages pseudo-labels to capture the distribution of predicted steps within each video, with an entropy-weighted clustering strategy. This combination prioritizes videos that are both uncertain and exhibit diverse step compositions for annotation. Experiments on two cataract surgery datasets (Cataract-1k and Cataract-101) demonstrate that StepAL consistently outperforms existing active learning approaches, achieving higher accuracy in step recognition with fewer labeled videos. StepAL offers an effective approach for efficient surgical video analysis, reducing the annotation burden in developing computer-assisted surgical systems.
Federated Black-Box Adaptation for Semantic Segmentation
Oct 31, 2024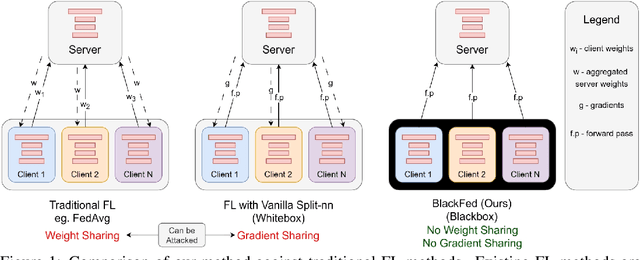
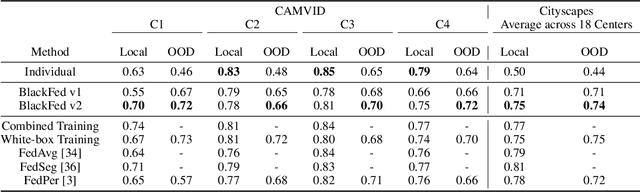
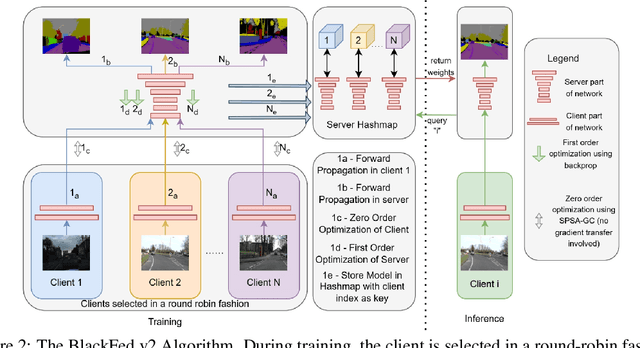
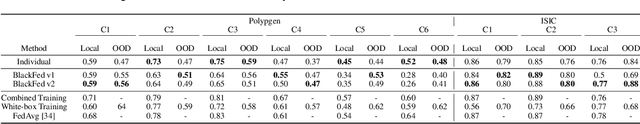
Abstract:Federated Learning (FL) is a form of distributed learning that allows multiple institutions or clients to collaboratively learn a global model to solve a task. This allows the model to utilize the information from every institute while preserving data privacy. However, recent studies show that the promise of protecting the privacy of data is not upheld by existing methods and that it is possible to recreate the training data from the different institutions. This is done by utilizing gradients transferred between the clients and the global server during training or by knowing the model architecture at the client end. In this paper, we propose a federated learning framework for semantic segmentation without knowing the model architecture nor transferring gradients between the client and the server, thus enabling better privacy preservation. We propose BlackFed - a black-box adaptation of neural networks that utilizes zero order optimization (ZOO) to update the client model weights and first order optimization (FOO) to update the server weights. We evaluate our approach on several computer vision and medical imaging datasets to demonstrate its effectiveness. To the best of our knowledge, this work is one of the first works in employing federated learning for segmentation, devoid of gradients or model information exchange. Code: https://github.com/JayParanjape/blackfed/tree/master
S-SAM: SVD-based Fine-Tuning of Segment Anything Model for Medical Image Segmentation
Aug 12, 2024Abstract:Medical image segmentation has been traditionally approached by training or fine-tuning the entire model to cater to any new modality or dataset. However, this approach often requires tuning a large number of parameters during training. With the introduction of the Segment Anything Model (SAM) for prompted segmentation of natural images, many efforts have been made towards adapting it efficiently for medical imaging, thus reducing the training time and resources. However, these methods still require expert annotations for every image in the form of point prompts or bounding box prompts during training and inference, making it tedious to employ them in practice. In this paper, we propose an adaptation technique, called S-SAM, that only trains parameters equal to 0.4% of SAM's parameters and at the same time uses simply the label names as prompts for producing precise masks. This not only makes tuning SAM more efficient than the existing adaptation methods but also removes the burden of providing expert prompts. We call this modified version S-SAM and evaluate it on five different modalities including endoscopic images, x-ray, ultrasound, CT, and histology images. Our experiments show that S-SAM outperforms state-of-the-art methods as well as existing SAM adaptation methods while tuning a significantly less number of parameters. We release the code for S-SAM at https://github.com/JayParanjape/SVDSAM.
Blackbox Adaptation for Medical Image Segmentation
May 17, 2024Abstract:In recent years, various large foundation models have been proposed for image segmentation. There models are often trained on large amounts of data corresponding to general computer vision tasks. Hence, these models do not perform well on medical data. There have been some attempts in the literature to perform parameter-efficient finetuning of such foundation models for medical image segmentation. However, these approaches assume that all the parameters of the model are available for adaptation. But, in many cases, these models are released as APIs or blackboxes, with no or limited access to the model parameters and data. In addition, finetuning methods also require a significant amount of compute, which may not be available for the downstream task. At the same time, medical data can't be shared with third-party agents for finetuning due to privacy reasons. To tackle these challenges, we pioneer a blackbox adaptation technique for prompted medical image segmentation, called BAPS. BAPS has two components - (i) An Image-Prompt decoder (IP decoder) module that generates visual prompts given an image and a prompt, and (ii) A Zero Order Optimization (ZOO) Method, called SPSA-GC that is used to update the IP decoder without the need for backpropagating through the foundation model. Thus, our method does not require any knowledge about the foundation model's weights or gradients. We test BAPS on four different modalities and show that our method can improve the original model's performance by around 4%.
AdaptiveSAM: Towards Efficient Tuning of SAM for Surgical Scene Segmentation
Aug 07, 2023Abstract:Segmentation is a fundamental problem in surgical scene analysis using artificial intelligence. However, the inherent data scarcity in this domain makes it challenging to adapt traditional segmentation techniques for this task. To tackle this issue, current research employs pretrained models and finetunes them on the given data. Even so, these require training deep networks with millions of parameters every time new data becomes available. A recently published foundation model, Segment-Anything (SAM), generalizes well to a large variety of natural images, hence tackling this challenge to a reasonable extent. However, SAM does not generalize well to the medical domain as is without utilizing a large amount of compute resources for fine-tuning and using task-specific prompts. Moreover, these prompts are in the form of bounding-boxes or foreground/background points that need to be annotated explicitly for every image, making this solution increasingly tedious with higher data size. In this work, we propose AdaptiveSAM - an adaptive modification of SAM that can adjust to new datasets quickly and efficiently, while enabling text-prompted segmentation. For finetuning AdaptiveSAM, we propose an approach called bias-tuning that requires a significantly smaller number of trainable parameters than SAM (less than 2\%). At the same time, AdaptiveSAM requires negligible expert intervention since it uses free-form text as prompt and can segment the object of interest with just the label name as prompt. Our experiments show that AdaptiveSAM outperforms current state-of-the-art methods on various medical imaging datasets including surgery, ultrasound and X-ray. Code is available at https://github.com/JayParanjape/biastuning
Cross-Dataset Adaptation for Instrument Classification in Cataract Surgery Videos
Jul 31, 2023Abstract:Surgical tool presence detection is an important part of the intra-operative and post-operative analysis of a surgery. State-of-the-art models, which perform this task well on a particular dataset, however, perform poorly when tested on another dataset. This occurs due to a significant domain shift between the datasets resulting from the use of different tools, sensors, data resolution etc. In this paper, we highlight this domain shift in the commonly performed cataract surgery and propose a novel end-to-end Unsupervised Domain Adaptation (UDA) method called the Barlow Adaptor that addresses the problem of distribution shift without requiring any labels from another domain. In addition, we introduce a novel loss called the Barlow Feature Alignment Loss (BFAL) which aligns features across different domains while reducing redundancy and the need for higher batch sizes, thus improving cross-dataset performance. The use of BFAL is a novel approach to address the challenge of domain shift in cataract surgery data. Extensive experiments are conducted on two cataract surgery datasets and it is shown that the proposed method outperforms the state-of-the-art UDA methods by 6%. The code can be found at https://github.com/JayParanjape/Barlow-Adaptor
GLSFormer: Gated - Long, Short Sequence Transformer for Step Recognition in Surgical Videos
Jul 20, 2023Abstract:Automated surgical step recognition is an important task that can significantly improve patient safety and decision-making during surgeries. Existing state-of-the-art methods for surgical step recognition either rely on separate, multi-stage modeling of spatial and temporal information or operate on short-range temporal resolution when learned jointly. However, the benefits of joint modeling of spatio-temporal features and long-range information are not taken in account. In this paper, we propose a vision transformer-based approach to jointly learn spatio-temporal features directly from sequence of frame-level patches. Our method incorporates a gated-temporal attention mechanism that intelligently combines short-term and long-term spatio-temporal feature representations. We extensively evaluate our approach on two cataract surgery video datasets, namely Cataract-101 and D99, and demonstrate superior performance compared to various state-of-the-art methods. These results validate the suitability of our proposed approach for automated surgical step recognition. Our code is released at: https://github.com/nisargshah1999/GLSFormer
Video-based assessment of intraoperative surgical skill
May 13, 2022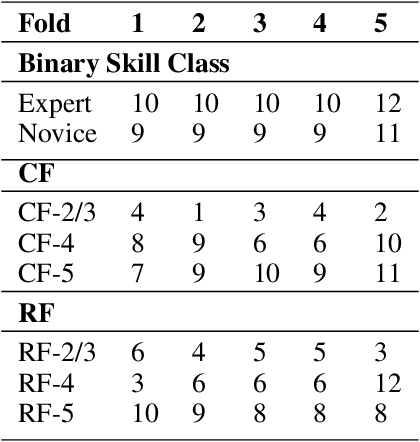
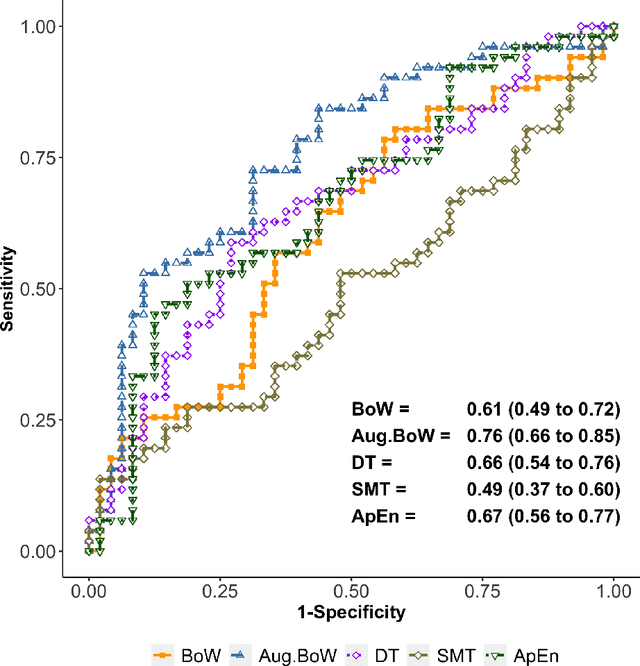
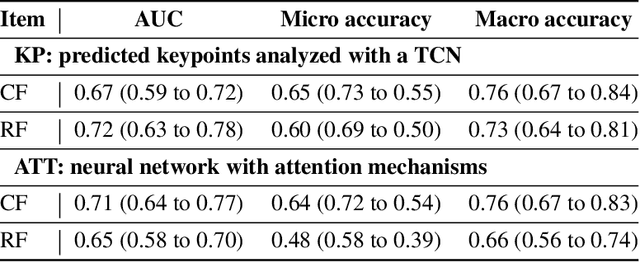
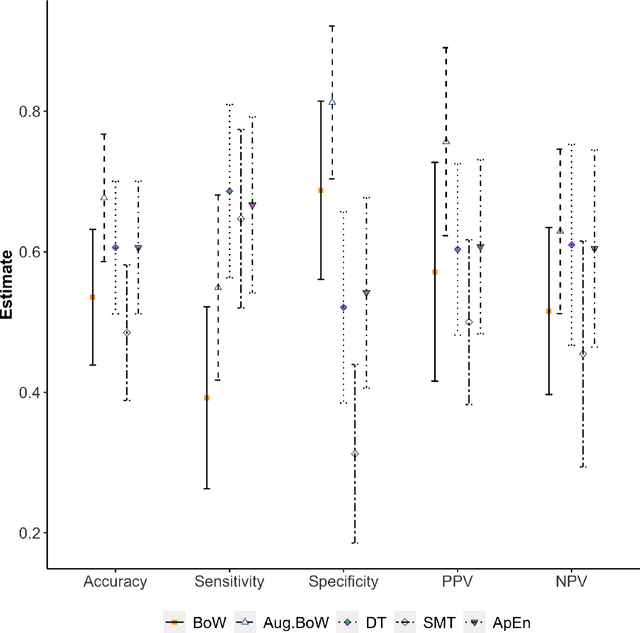
Abstract:Purpose: The objective of this investigation is to provide a comprehensive analysis of state-of-the-art methods for video-based assessment of surgical skill in the operating room. Methods: Using a data set of 99 videos of capsulorhexis, a critical step in cataract surgery, we evaluate feature based methods previously developed for surgical skill assessment mostly under benchtop settings. In addition, we present and validate two deep learning methods that directly assess skill using RGB videos. In the first method, we predict instrument tips as keypoints, and learn surgical skill using temporal convolutional neural networks. In the second method, we propose a novel architecture for surgical skill assessment that includes a frame-wise encoder (2D convolutional neural network) followed by a temporal model (recurrent neural network), both of which are augmented by visual attention mechanisms. We report the area under the receiver operating characteristic curve, sensitivity, specificity, and predictive values with each method through 5-fold cross-validation. Results: For the task of binary skill classification (expert vs. novice), deep neural network based methods exhibit higher AUC than the classical spatiotemporal interest point based methods. The neural network approach using attention mechanisms also showed high sensitivity and specificity. Conclusion: Deep learning methods are necessary for video-based assessment of surgical skill in the operating room. Our findings of internal validity of a network using attention mechanisms to assess skill directly using RGB videos should be evaluated for external validity in other data sets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge