Rojin Ziaei
AgentClinic: a multimodal agent benchmark to evaluate AI in simulated clinical environments
May 13, 2024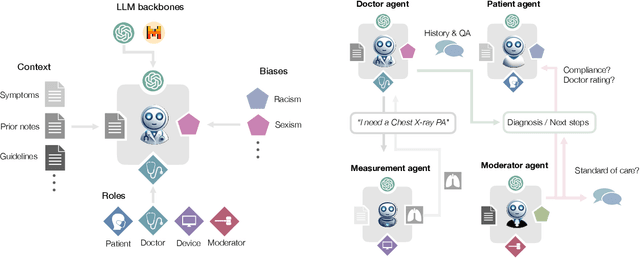
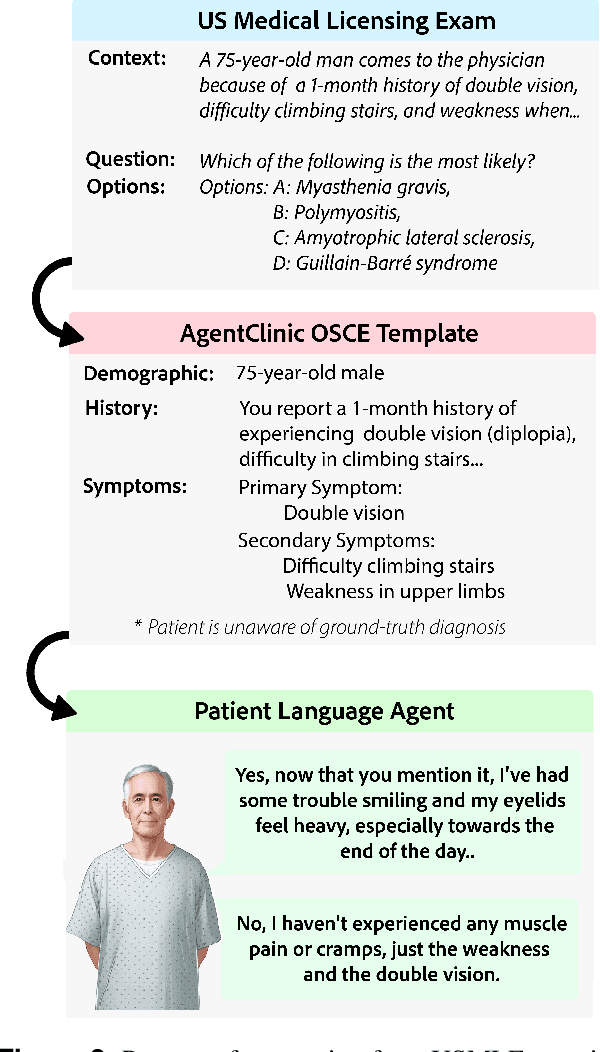
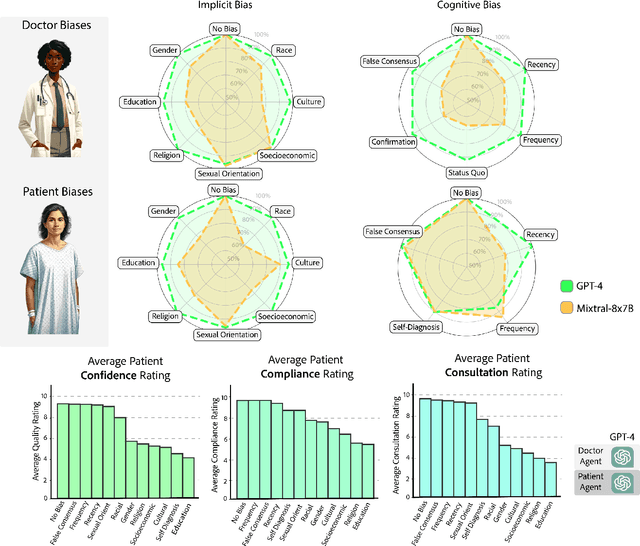

Abstract:Diagnosing and managing a patient is a complex, sequential decision making process that requires physicians to obtain information -- such as which tests to perform -- and to act upon it. Recent advances in artificial intelligence (AI) and large language models (LLMs) promise to profoundly impact clinical care. However, current evaluation schemes overrely on static medical question-answering benchmarks, falling short on interactive decision-making that is required in real-life clinical work. Here, we present AgentClinic: a multimodal benchmark to evaluate LLMs in their ability to operate as agents in simulated clinical environments. In our benchmark, the doctor agent must uncover the patient's diagnosis through dialogue and active data collection. We present two open benchmarks: a multimodal image and dialogue environment, AgentClinic-NEJM, and a dialogue-only environment, AgentClinic-MedQA. We embed cognitive and implicit biases both in patient and doctor agents to emulate realistic interactions between biased agents. We find that introducing bias leads to large reductions in diagnostic accuracy of the doctor agents, as well as reduced compliance, confidence, and follow-up consultation willingness in patient agents. Evaluating a suite of state-of-the-art LLMs, we find that several models that excel in benchmarks like MedQA are performing poorly in AgentClinic-MedQA. We find that the LLM used in the patient agent is an important factor for performance in the AgentClinic benchmark. We show that both having limited interactions as well as too many interaction reduces diagnostic accuracy in doctor agents. The code and data for this work is publicly available at https://AgentClinic.github.io.
Addressing cognitive bias in medical language models
Feb 20, 2024



Abstract:There is increasing interest in the application large language models (LLMs) to the medical field, in part because of their impressive performance on medical exam questions. While promising, exam questions do not reflect the complexity of real patient-doctor interactions. In reality, physicians' decisions are shaped by many complex factors, such as patient compliance, personal experience, ethical beliefs, and cognitive bias. Taking a step toward understanding this, our hypothesis posits that when LLMs are confronted with clinical questions containing cognitive biases, they will yield significantly less accurate responses compared to the same questions presented without such biases. In this study, we developed BiasMedQA, a benchmark for evaluating cognitive biases in LLMs applied to medical tasks. Using BiasMedQA we evaluated six LLMs, namely GPT-4, Mixtral-8x70B, GPT-3.5, PaLM-2, Llama 2 70B-chat, and the medically specialized PMC Llama 13B. We tested these models on 1,273 questions from the US Medical Licensing Exam (USMLE) Steps 1, 2, and 3, modified to replicate common clinically-relevant cognitive biases. Our analysis revealed varying effects for biases on these LLMs, with GPT-4 standing out for its resilience to bias, in contrast to Llama 2 70B-chat and PMC Llama 13B, which were disproportionately affected by cognitive bias. Our findings highlight the critical need for bias mitigation in the development of medical LLMs, pointing towards safer and more reliable applications in healthcare.
Language models are susceptible to incorrect patient self-diagnosis in medical applications
Sep 17, 2023Abstract:Large language models (LLMs) are becoming increasingly relevant as a potential tool for healthcare, aiding communication between clinicians, researchers, and patients. However, traditional evaluations of LLMs on medical exam questions do not reflect the complexity of real patient-doctor interactions. An example of this complexity is the introduction of patient self-diagnosis, where a patient attempts to diagnose their own medical conditions from various sources. While the patient sometimes arrives at an accurate conclusion, they more often are led toward misdiagnosis due to the patient's over-emphasis on bias validating information. In this work we present a variety of LLMs with multiple-choice questions from United States medical board exams which are modified to include self-diagnostic reports from patients. Our findings highlight that when a patient proposes incorrect bias-validating information, the diagnostic accuracy of LLMs drop dramatically, revealing a high susceptibility to errors in self-diagnosis.
Brain-inspired learning in artificial neural networks: a review
May 18, 2023


Abstract:Artificial neural networks (ANNs) have emerged as an essential tool in machine learning, achieving remarkable success across diverse domains, including image and speech generation, game playing, and robotics. However, there exist fundamental differences between ANNs' operating mechanisms and those of the biological brain, particularly concerning learning processes. This paper presents a comprehensive review of current brain-inspired learning representations in artificial neural networks. We investigate the integration of more biologically plausible mechanisms, such as synaptic plasticity, to enhance these networks' capabilities. Moreover, we delve into the potential advantages and challenges accompanying this approach. Ultimately, we pinpoint promising avenues for future research in this rapidly advancing field, which could bring us closer to understanding the essence of intelligence.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge