Robert Boutin
Data-Limited Tissue Segmentation using Inpainting-Based Self-Supervised Learning
Oct 14, 2022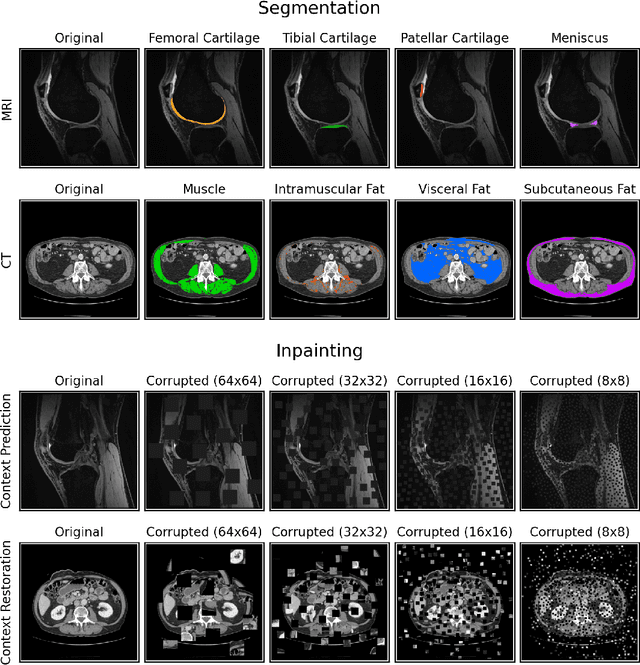
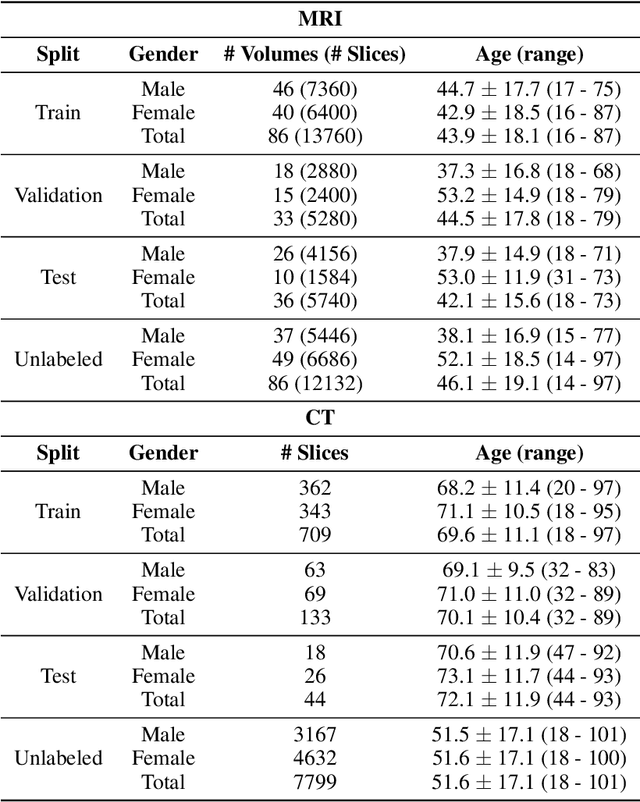
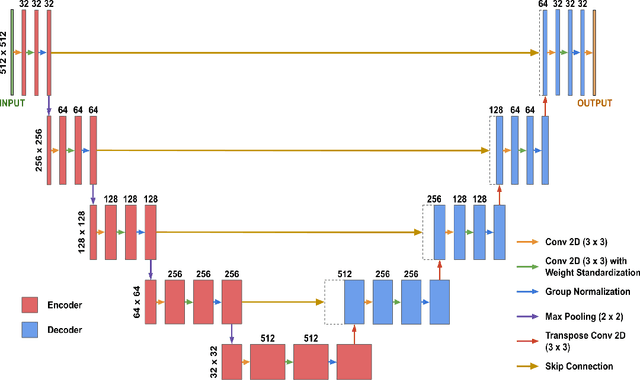
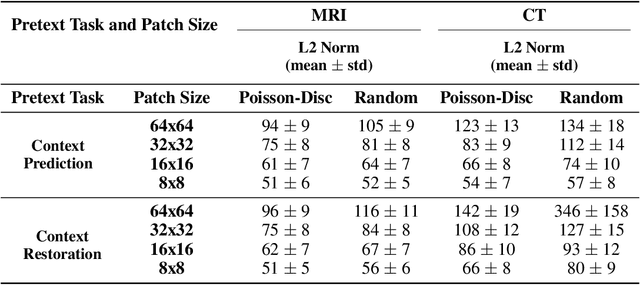
Abstract:Although supervised learning has enabled high performance for image segmentation, it requires a large amount of labeled training data, which can be difficult to obtain in the medical imaging field. Self-supervised learning (SSL) methods involving pretext tasks have shown promise in overcoming this requirement by first pretraining models using unlabeled data. In this work, we evaluate the efficacy of two SSL methods (inpainting-based pretext tasks of context prediction and context restoration) for CT and MRI image segmentation in label-limited scenarios, and investigate the effect of implementation design choices for SSL on downstream segmentation performance. We demonstrate that optimally trained and easy-to-implement inpainting-based SSL segmentation models can outperform classically supervised methods for MRI and CT tissue segmentation in label-limited scenarios, for both clinically-relevant metrics and the traditional Dice score.
SKM-TEA: A Dataset for Accelerated MRI Reconstruction with Dense Image Labels for Quantitative Clinical Evaluation
Mar 14, 2022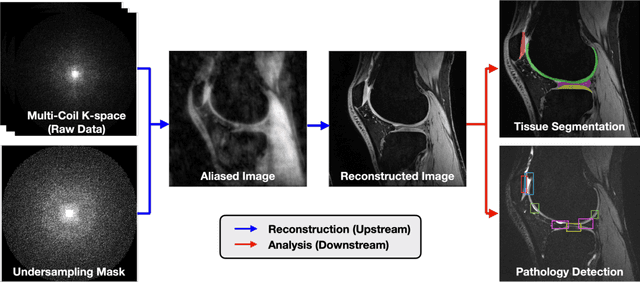
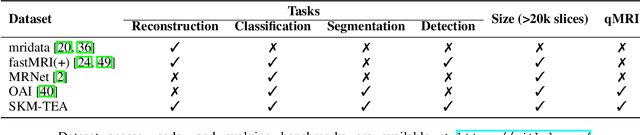
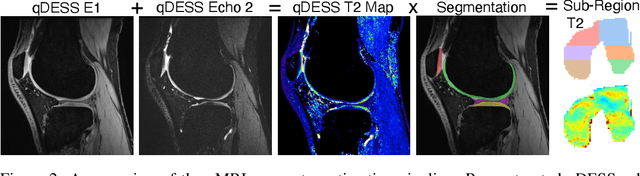
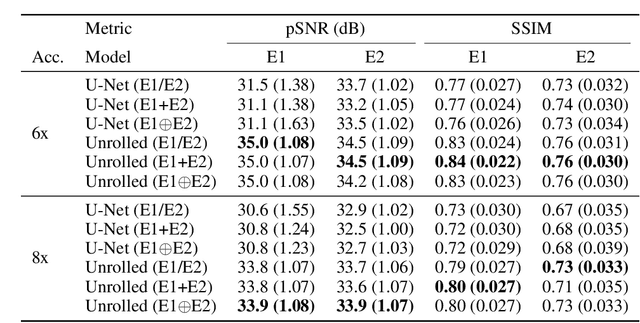
Abstract:Magnetic resonance imaging (MRI) is a cornerstone of modern medical imaging. However, long image acquisition times, the need for qualitative expert analysis, and the lack of (and difficulty extracting) quantitative indicators that are sensitive to tissue health have curtailed widespread clinical and research studies. While recent machine learning methods for MRI reconstruction and analysis have shown promise for reducing this burden, these techniques are primarily validated with imperfect image quality metrics, which are discordant with clinically-relevant measures that ultimately hamper clinical deployment and clinician trust. To mitigate this challenge, we present the Stanford Knee MRI with Multi-Task Evaluation (SKM-TEA) dataset, a collection of quantitative knee MRI (qMRI) scans that enables end-to-end, clinically-relevant evaluation of MRI reconstruction and analysis tools. This 1.6TB dataset consists of raw-data measurements of ~25,000 slices (155 patients) of anonymized patient MRI scans, the corresponding scanner-generated DICOM images, manual segmentations of four tissues, and bounding box annotations for sixteen clinically relevant pathologies. We provide a framework for using qMRI parameter maps, along with image reconstructions and dense image labels, for measuring the quality of qMRI biomarker estimates extracted from MRI reconstruction, segmentation, and detection techniques. Finally, we use this framework to benchmark state-of-the-art baselines on this dataset. We hope our SKM-TEA dataset and code can enable a broad spectrum of research for modular image reconstruction and image analysis in a clinically informed manner. Dataset access, code, and benchmarks are available at https://github.com/StanfordMIMI/skm-tea.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge