Rasoul Sali
HMIC: Hierarchical Medical Image Classification, A Deep Learning Approach
Jun 23, 2020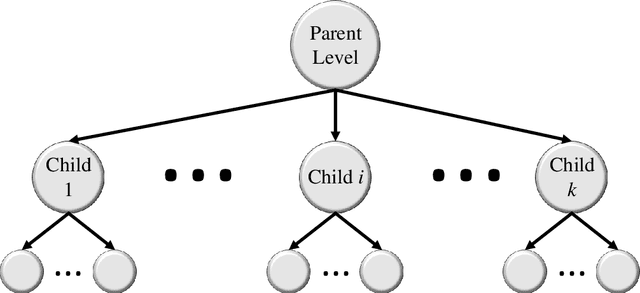
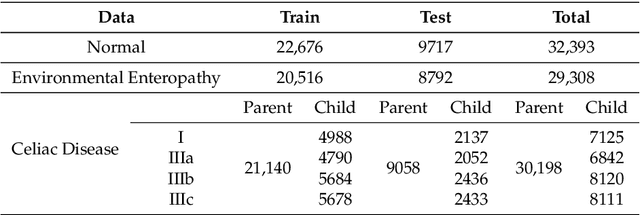
Abstract:Image classification is central to the big data revolution in medicine. Improved information processing methods for diagnosis and classification of digital medical images have shown to be successful via deep learning approaches. As this field is explored, there are limitations to the performance of traditional supervised classifiers. This paper outlines an approach that is different from the current medical image classification tasks that view the issue as multi-class classification. We performed a hierarchical classification using our Hierarchical Medical Image classification (HMIC) approach. HMIC uses stacks of deep learning models to give particular comprehension at each level of the clinical picture hierarchy. For testing our performance, we use biopsy of the small bowel images that contain three categories in the parent level (Celiac Disease, Environmental Enteropathy, and histologically normal controls). For the child level, Celiac Disease Severity is classified into 4 classes (I, IIIa, IIIb, and IIIC).
Hierarchical Deep Convolutional Neural Networks for Multi-category Diagnosis of Gastrointestinal Disorders on Histopathological Images
May 08, 2020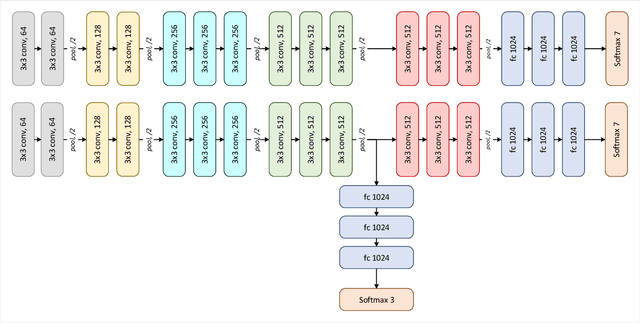
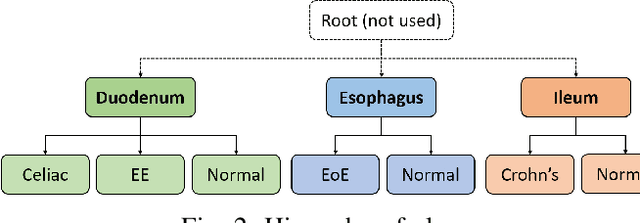
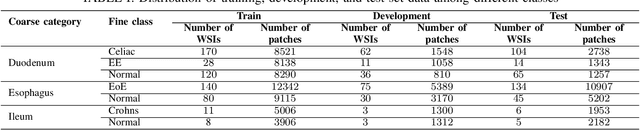
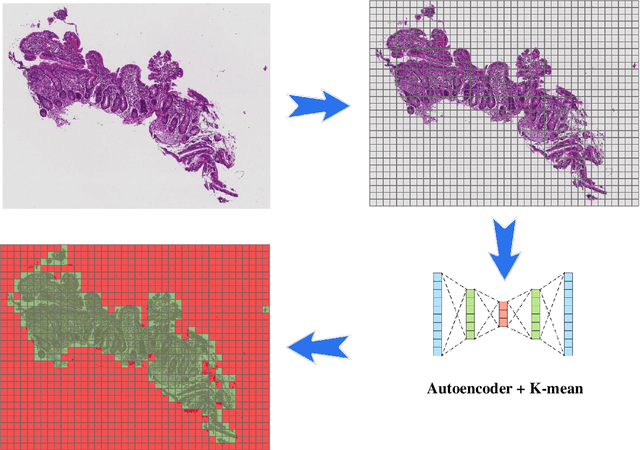
Abstract:Deep convolutional neural networks (CNNs) have been successful for a wide range of computer vision tasks including image classification. A specific area of application lies in digital pathology for pattern recognition in tissue-based diagnosis of gastrointestinal (GI) diseases. This domain can utilize CNNs to translate histopathological images into precise diagnostics. This is challenging since these complex biopsies are heterogeneous and require multiple levels of assessment. This is mainly due to structural similarities in different parts of the GI tract and shared features among different gut diseases. Addressing this problem with a flat model which assumes all classes (parts of the gut and their diseases) are equally difficult to distinguish leads to an inadequate assessment of each class. Since hierarchical model restricts classification error to each sub-class, it leads to a more informative model compared to a flat model. In this paper we propose to apply hierarchical classification of biopsy images from different parts of the GI tract and the receptive diseases within each. We embedded a class hierarchy into the plain VGGNet to take advantage of the hierarchical structure of its layers. The proposed model was evaluated using an independent set of image patches from 373 whole slide images. The results indicate that hierarchical model can achieve better results compared to the flat model for multi-category diagnosis of GI disorders using histopathological images.
CeliacNet: Celiac Disease Severity Diagnosis on Duodenal Histopathological Images Using Deep Residual Networks
Oct 07, 2019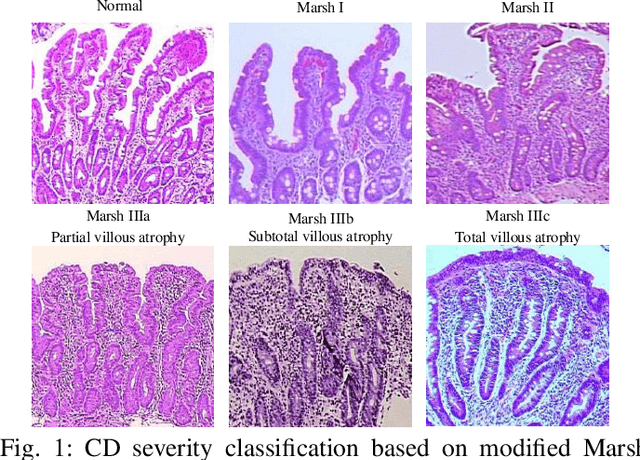
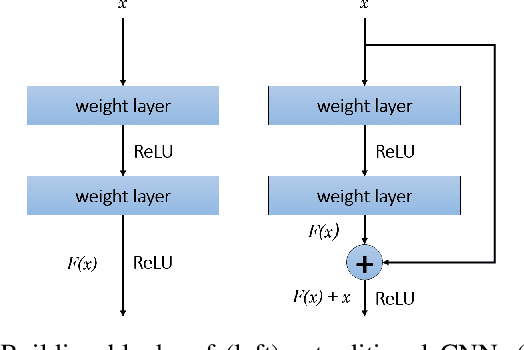
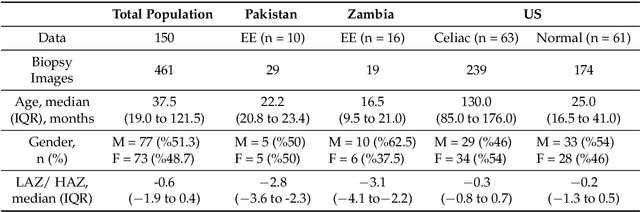
Abstract:Celiac Disease (CD) is a chronic autoimmune disease that affects the small intestine in genetically predisposed children and adults. Gluten exposure triggers an inflammatory cascade which leads to compromised intestinal barrier function. If this enteropathy is unrecognized, this can lead to anemia, decreased bone density, and, in longstanding cases, intestinal cancer. The prevalence of the disorder is 1% in the United States. An intestinal (duodenal) biopsy is considered the "gold standard" for diagnosis. The mild CD might go unnoticed due to non-specific clinical symptoms or mild histologic features. In our current work, we trained a model based on deep residual networks to diagnose CD severity using a histological scoring system called the modified Marsh score. The proposed model was evaluated using an independent set of 120 whole slide images from 15 CD patients and achieved an AUC greater than 0.96 in all classes. These results demonstrate the diagnostic power of the proposed model for CD severity classification using histological images.
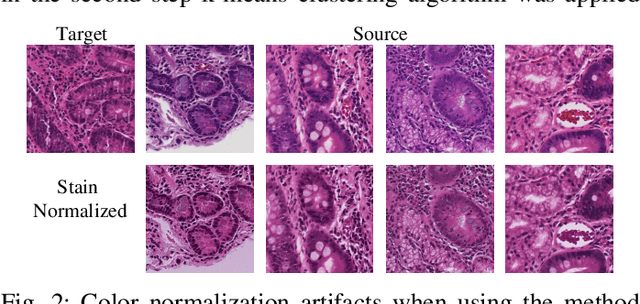
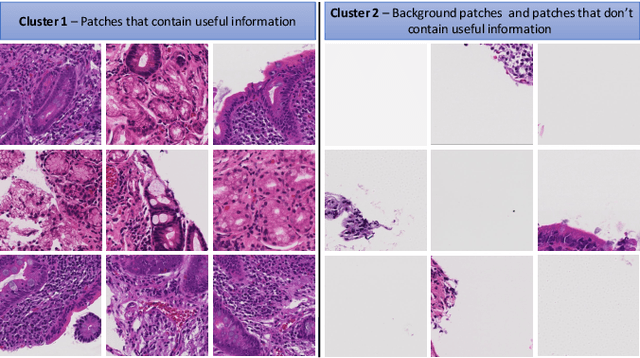
Diagnosis of Celiac Disease and Environmental Enteropathy on Biopsy Images Using Color Balancing on Convolutional Neural Networks
Apr 24, 2019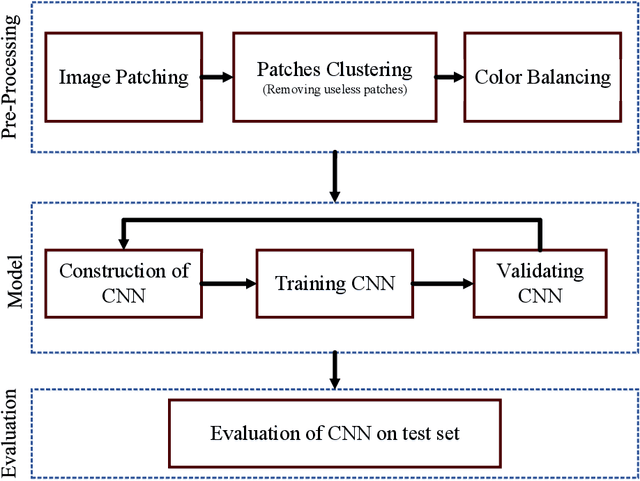
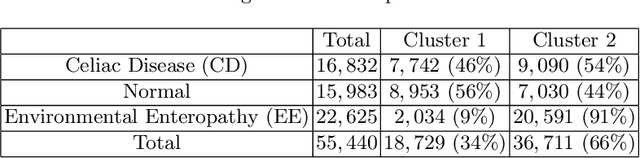
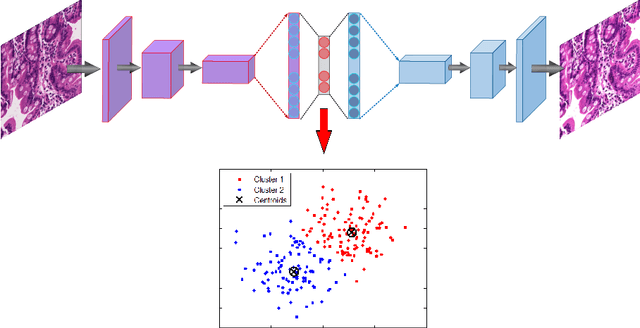
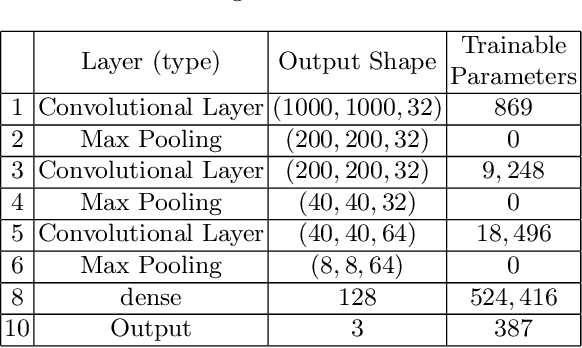
Abstract:Celiac Disease (CD) and Environmental Enteropathy (EE) are common causes of malnutrition and adversely impact normal childhood development. CD is an autoimmune disorder that is prevalent worldwide and is caused by an increased sensitivity to gluten. Gluten exposure destructs the small intestinal epithelial barrier, resulting in nutrient mal-absorption and childhood under-nutrition. EE also results in barrier dysfunction but is thought to be caused by an increased vulnerability to infections. EE has been implicated as the predominant cause of under-nutrition, oral vaccine failure, and impaired cognitive development in low-and-middle-income countries. Both conditions require a tissue biopsy for diagnosis, and a major challenge of interpreting clinical biopsy images to differentiate between these gastrointestinal diseases is striking histopathologic overlap between them. In the current study, we propose a convolutional neural network (CNN) to classify duodenal biopsy images from subjects with CD, EE, and healthy controls. We evaluated the performance of our proposed model using a large cohort containing 1000 biopsy images. Our evaluations show that the proposed model achieves an area under ROC of 0.99, 1.00, and 0.97 for CD, EE, and healthy controls, respectively. These results demonstrate the discriminative power of the proposed model in duodenal biopsies classification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge