Philipp Kickingereder
Deep Probabilistic Modeling of Glioma Growth
Jul 09, 2019


Abstract:Existing approaches to modeling the dynamics of brain tumor growth, specifically glioma, employ biologically inspired models of cell diffusion, using image data to estimate the associated parameters. In this work, we propose an alternative approach based on recent advances in probabilistic segmentation and representation learning that implicitly learns growth dynamics directly from data without an underlying explicit model. We present evidence that our approach is able to learn a distribution of plausible future tumor appearances conditioned on past observations of the same tumor.
Automated brain extraction of multi-sequence MRI using artificial neural networks
Jan 31, 2019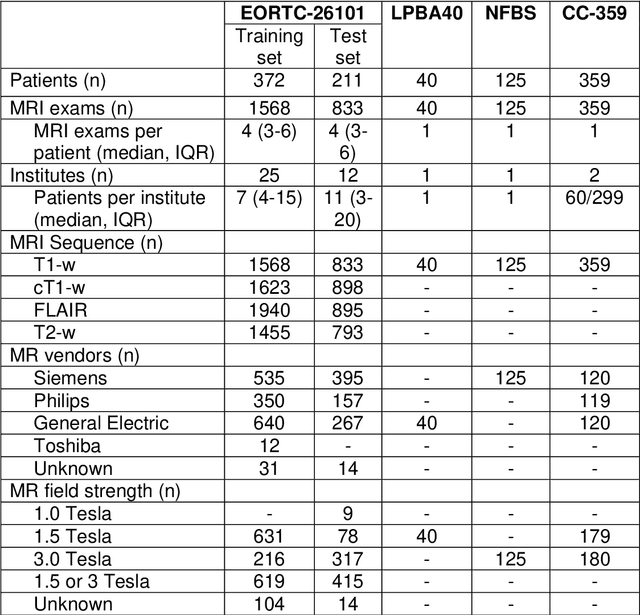
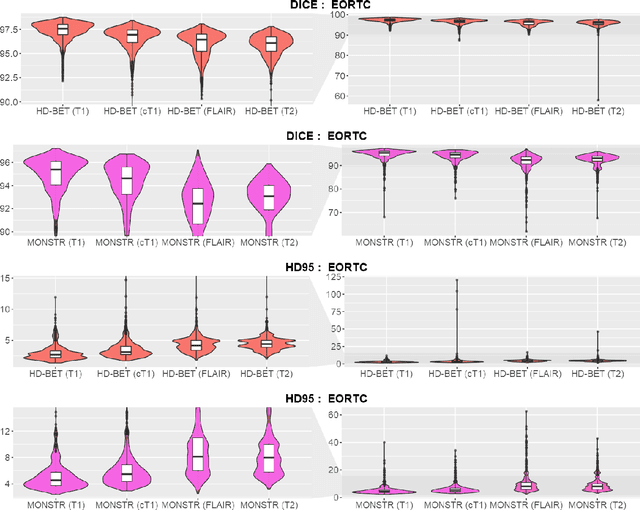
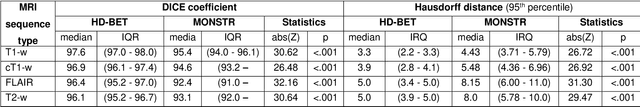
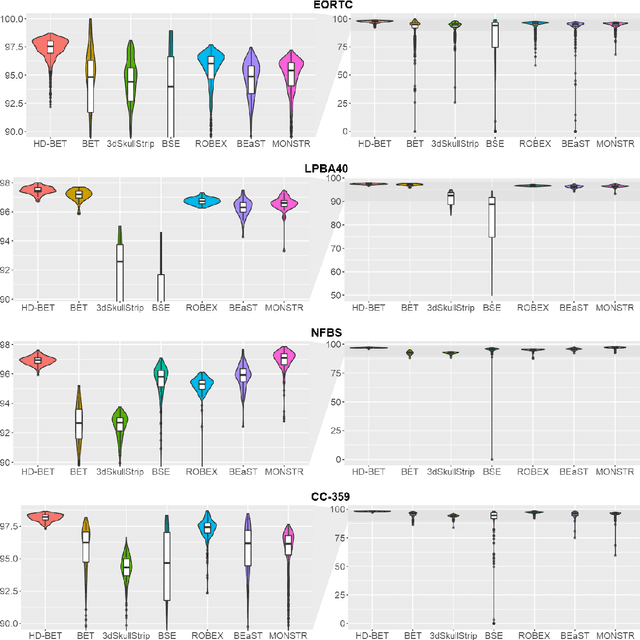
Abstract:Brain extraction is a critical preprocessing step in the analysis of MRI neuroimaging studies and influences the accuracy of downstream analyses. State-of-the-art brain extraction algorithms are, however, optimized for processing healthy brains and thus frequently fail in the presence of pathologically altered brain or when applied to heterogeneous MRI datasets. Here we introduce a new, rigorously validated algorithm (termed HD-BET) relying on artificial neural networks that aims to overcome these limitations. We demonstrate that HD-BET outperforms five publicly available state-of-the-art brain extraction algorithms in several large-scale neuroimaging datasets, including one from a prospective multicentric trial in neuro-oncology, yielding median improvements of +1.33 to +2.63 points for the DICE coefficient and -0.80 to -2.75 mm for the Hausdorff distance (Bonferroni-adjusted p<0.001). Importantly, the HD-BET algorithm shows robust performance in the presence of pathology or treatment-induced tissue alterations, is applicable to a broad range of MRI sequence types and is not influenced by variations in MRI hardware and acquisition parameters encountered in both research and clinical practice. For broader accessibility our HD-BET prediction algorithm is made freely available and may become an essential component for robust, automated, high-throughput processing of MRI neuroimaging data.
No New-Net
Sep 27, 2018
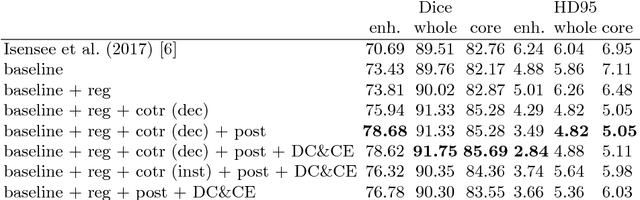
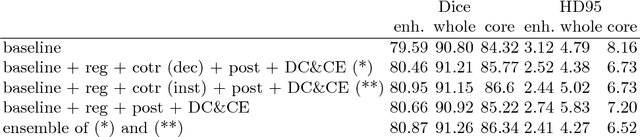

Abstract:In this paper we demonstrate the effectiveness of a well trained U-Net in the context of the BraTS 2018 challenge. This endeavour is particularly interesting given that researchers are currently besting each other with architectural modifications that are intended to improve the segmentation performance. We instead focus on the training process, argue that a well trained U-Net is hard to beat and intend to back up this assumption with a strong participation in this years BraTS challenge. Our baseline U-Net, which has only minor modifications and is trained with a large patch size and a dice loss function already achieves competitive dice scores on the BraTS2018 validation data. By incorporating region based training, additional training data and a simple postprocessing technique, we obtain dice scores of 81.01, 90.83 and 85.44 and Hausdorff Distances (95th percentile) of 2.54, 4.97 and 7.
Brain Tumor Segmentation and Radiomics Survival Prediction: Contribution to the BRATS 2017 Challenge
Feb 28, 2018
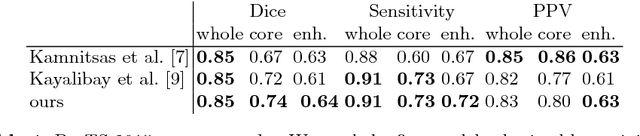

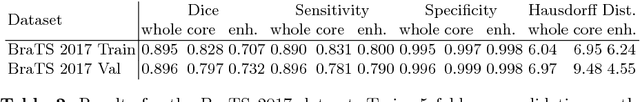
Abstract:Quantitative analysis of brain tumors is critical for clinical decision making. While manual segmentation is tedious, time consuming and subjective, this task is at the same time very challenging to solve for automatic segmentation methods. In this paper we present our most recent effort on developing a robust segmentation algorithm in the form of a convolutional neural network. Our network architecture was inspired by the popular U-Net and has been carefully modified to maximize brain tumor segmentation performance. We use a dice loss function to cope with class imbalances and use extensive data augmentation to successfully prevent overfitting. Our method beats the current state of the art on BraTS 2015, is one of the leading methods on the BraTS 2017 validation set (dice scores of 0.896, 0.797 and 0.732 for whole tumor, tumor core and enhancing tumor, respectively) and achieves very good Dice scores on the test set (0.858 for whole, 0.775 for core and 0.647 for enhancing tumor). We furthermore take part in the survival prediction subchallenge by training an ensemble of a random forest regressor and multilayer perceptrons on shape features describing the tumor subregions. Our approach achieves 52.6% accuracy, a Spearman correlation coefficient of 0.496 and a mean square error of 209607 on the test set.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge