Olivier Janssens
Overly Optimistic Prediction Results on Imbalanced Data: Flaws and Benefits of Applying Over-sampling
Jan 15, 2020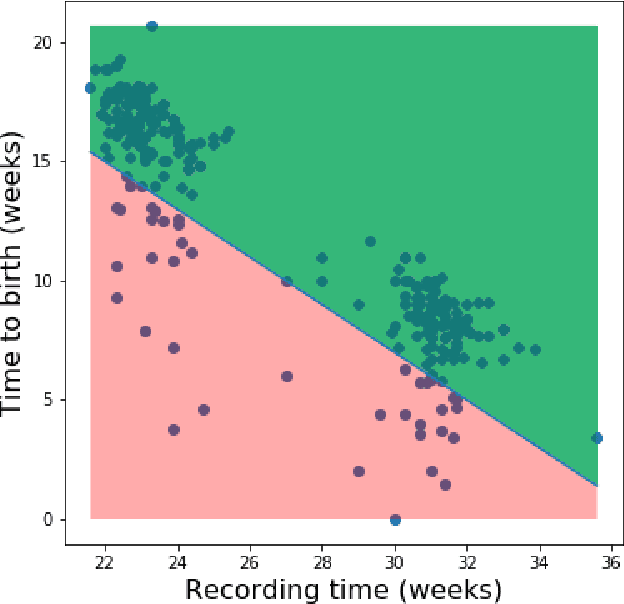
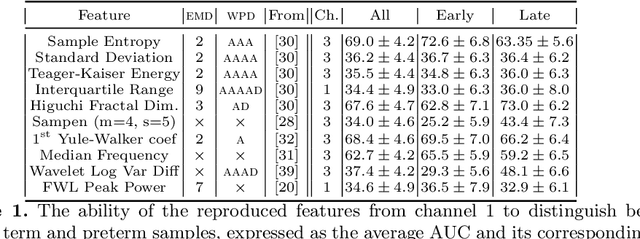
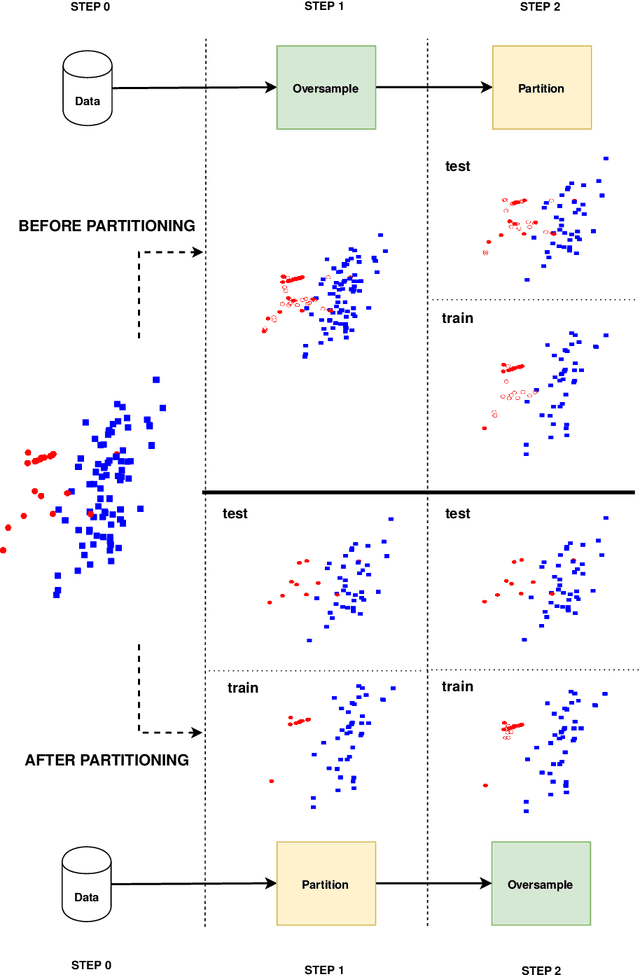
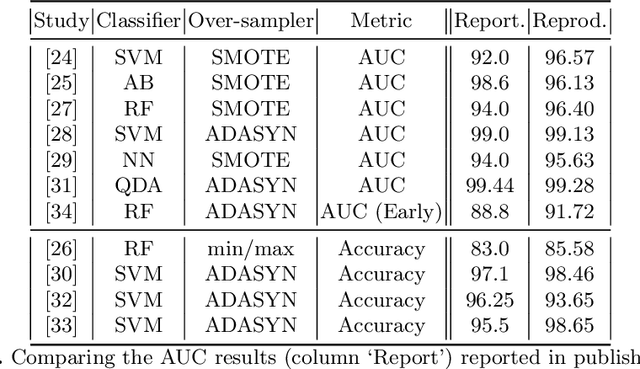
Abstract:Information extracted from electrohysterography recordings could potentially prove to be an interesting additional source of information to estimate the risk on preterm birth. Recently, a large number of studies have reported near-perfect results to distinguish between recordings of patients that will deliver term or preterm using a public resource, called the Term/Preterm Electrohysterogram database. However, we argue that these results are overly optimistic due to a methodological flaw being made. In this work, we focus on one specific type of methodological flaw: applying over-sampling before partitioning the data into mutually exclusive training and testing sets. We show how this causes the results to be biased using two artificial datasets and reproduce results of studies in which this flaw was identified. Moreover, we evaluate the actual impact of over-sampling on predictive performance, when applied prior to data partitioning, using the same methodologies of related studies, to provide a realistic view of these methodologies' generalization capabilities. We make our research reproducible by providing all the code under an open license.
Web Applicable Computer-aided Diagnosis of Glaucoma Using Deep Learning
Dec 06, 2018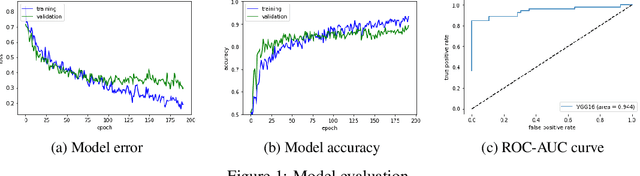

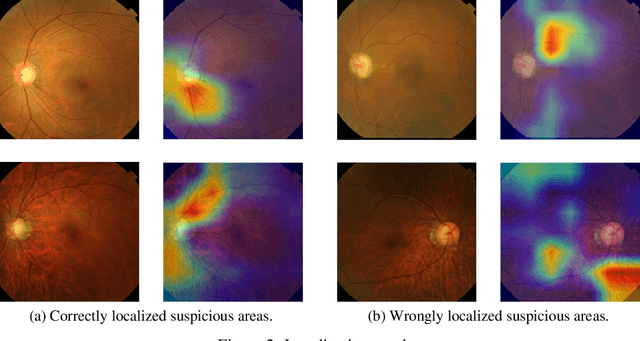
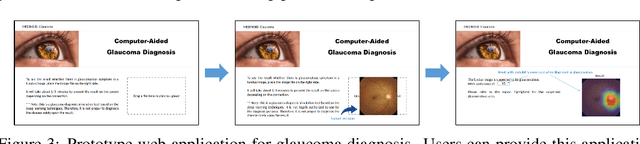
Abstract:Glaucoma is a major eye disease, leading to vision loss in the absence of proper medical treatment. Current diagnosis of glaucoma is performed by ophthalmologists who are often analyzing several types of medical images generated by different types of medical equipment. Capturing and analyzing these medical images is labor-intensive and expensive. In this paper, we present a novel computational approach towards glaucoma diagnosis and localization, only making use of eye fundus images that are analyzed by state-of-the-art deep learning techniques. Specifically, our approach leverages Convolutional Neural Networks (CNNs) and Gradient-weighted Class Activation Mapping (Grad-CAM) for glaucoma diagnosis and localization, respectively. Quantitative and qualitative results, as obtained for a small-sized dataset with no segmentation ground truth, demonstrate that the proposed approach is promising, for instance achieving an accuracy of 0.91$\pm0.02$ and an ROC-AUC score of 0.94 for the diagnosis task. Furthermore, we present a publicly available prototype web application that integrates our predictive model, with the goal of making effective glaucoma diagnosis available to a wide audience.
GENESIM: genetic extraction of a single, interpretable model
Nov 17, 2016

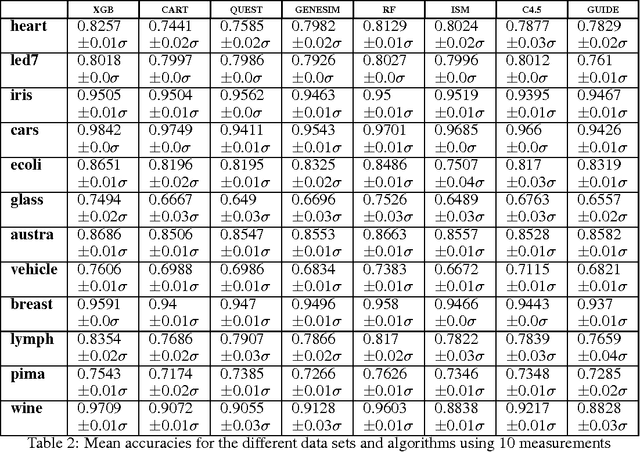
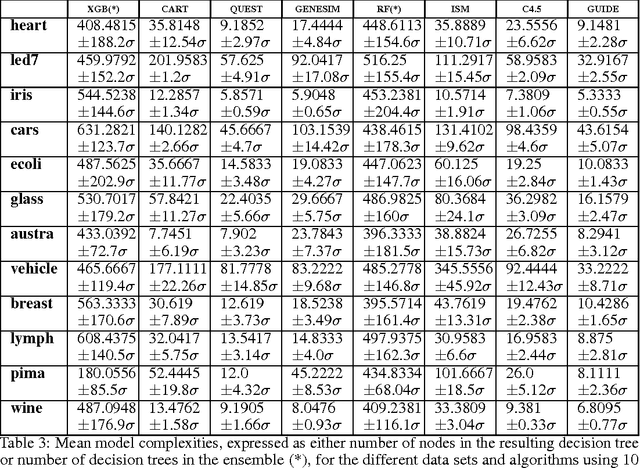
Abstract:Models obtained by decision tree induction techniques excel in being interpretable.However, they can be prone to overfitting, which results in a low predictive performance. Ensemble techniques are able to achieve a higher accuracy. However, this comes at a cost of losing interpretability of the resulting model. This makes ensemble techniques impractical in applications where decision support, instead of decision making, is crucial. To bridge this gap, we present the GENESIM algorithm that transforms an ensemble of decision trees to a single decision tree with an enhanced predictive performance by using a genetic algorithm. We compared GENESIM to prevalent decision tree induction and ensemble techniques using twelve publicly available data sets. The results show that GENESIM achieves a better predictive performance on most of these data sets than decision tree induction techniques and a predictive performance in the same order of magnitude as the ensemble techniques. Moreover, the resulting model of GENESIM has a very low complexity, making it very interpretable, in contrast to ensemble techniques.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge