Noussair Lazrak
medigan: A Python Library of Pretrained Generative Models for Enriched Data Access in Medical Imaging
Sep 28, 2022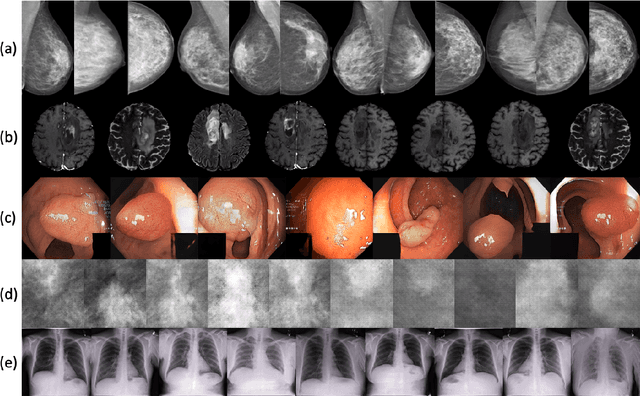
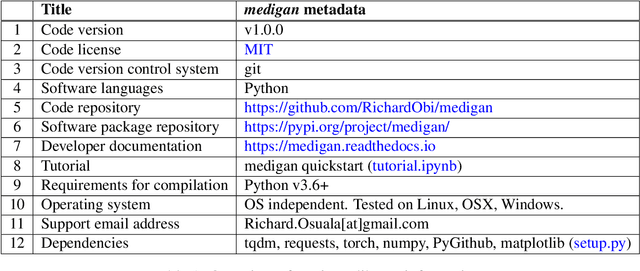
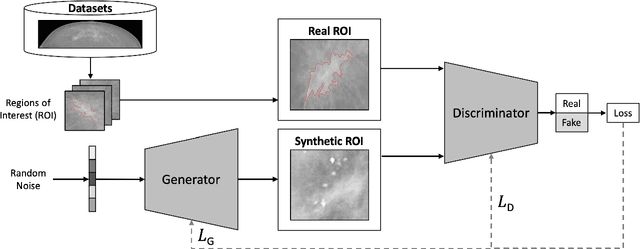
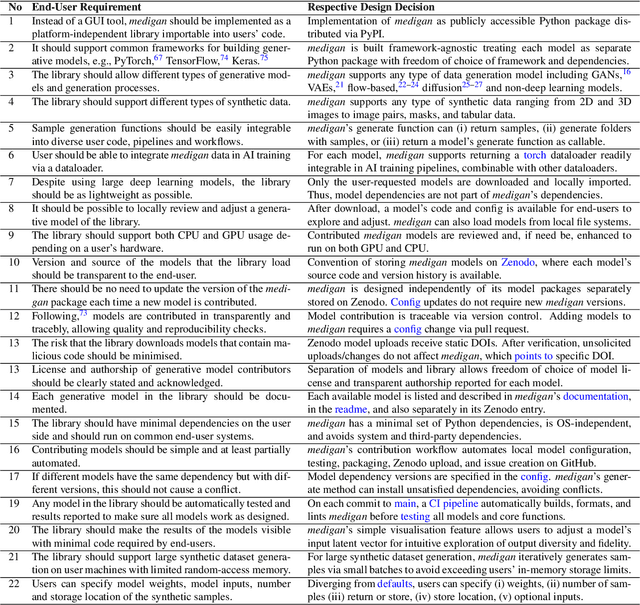
Abstract:Synthetic data generated by generative models can enhance the performance and capabilities of data-hungry deep learning models in medical imaging. However, there is (1) limited availability of (synthetic) datasets and (2) generative models are complex to train, which hinders their adoption in research and clinical applications. To reduce this entry barrier, we propose medigan, a one-stop shop for pretrained generative models implemented as an open-source framework-agnostic Python library. medigan allows researchers and developers to create, increase, and domain-adapt their training data in just a few lines of code. Guided by design decisions based on gathered end-user requirements, we implement medigan based on modular components for generative model (i) execution, (ii) visualisation, (iii) search & ranking, and (iv) contribution. The library's scalability and design is demonstrated by its growing number of integrated and readily-usable pretrained generative models consisting of 21 models utilising 9 different Generative Adversarial Network architectures trained on 11 datasets from 4 domains, namely, mammography, endoscopy, x-ray, and MRI. Furthermore, 3 applications of medigan are analysed in this work, which include (a) enabling community-wide sharing of restricted data, (b) investigating generative model evaluation metrics, and (c) improving clinical downstream tasks. In (b), extending on common medical image synthesis assessment and reporting standards, we show Fr\'echet Inception Distance variability based on image normalisation and radiology-specific feature extraction.
FUTURE-AI: Guiding Principles and Consensus Recommendations for Trustworthy Artificial Intelligence in Medical Imaging
Sep 29, 2021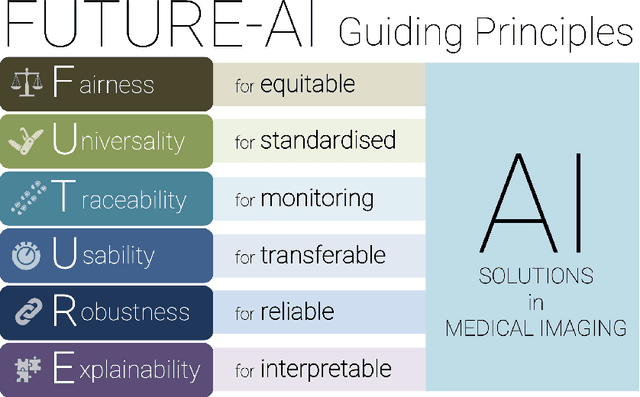
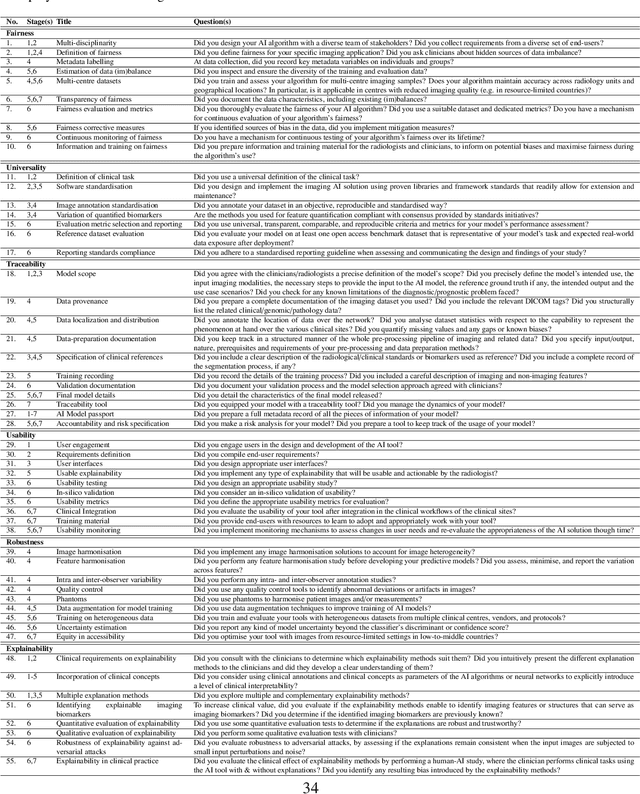
Abstract:The recent advancements in artificial intelligence (AI) combined with the extensive amount of data generated by today's clinical systems, has led to the development of imaging AI solutions across the whole value chain of medical imaging, including image reconstruction, medical image segmentation, image-based diagnosis and treatment planning. Notwithstanding the successes and future potential of AI in medical imaging, many stakeholders are concerned of the potential risks and ethical implications of imaging AI solutions, which are perceived as complex, opaque, and difficult to comprehend, utilise, and trust in critical clinical applications. Despite these concerns and risks, there are currently no concrete guidelines and best practices for guiding future AI developments in medical imaging towards increased trust, safety and adoption. To bridge this gap, this paper introduces a careful selection of guiding principles drawn from the accumulated experiences, consensus, and best practices from five large European projects on AI in Health Imaging. These guiding principles are named FUTURE-AI and its building blocks consist of (i) Fairness, (ii) Universality, (iii) Traceability, (iv) Usability, (v) Robustness and (vi) Explainability. In a step-by-step approach, these guidelines are further translated into a framework of concrete recommendations for specifying, developing, evaluating, and deploying technically, clinically and ethically trustworthy AI solutions into clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge