Michael D. Abramoff
Automated segmentation of choroidal layers from 3-dimensional macular optical coherence tomography scans
Mar 11, 2021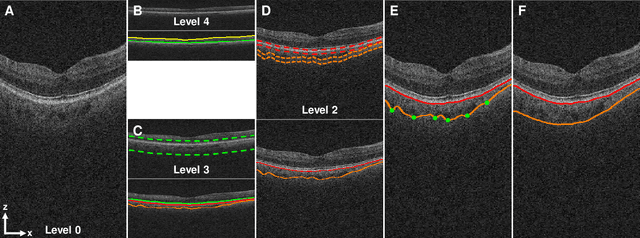
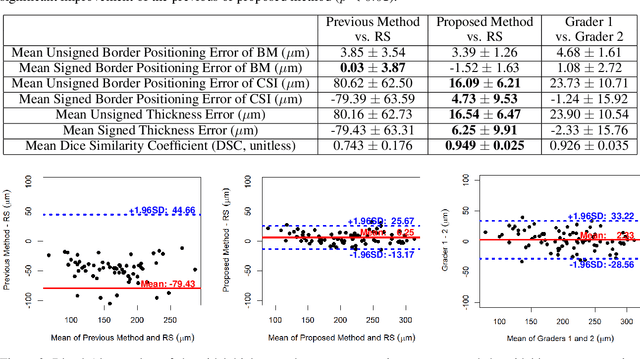
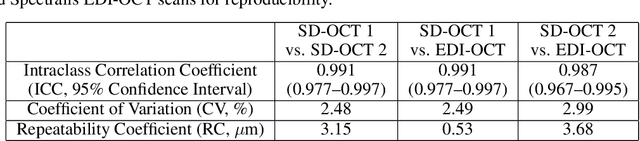
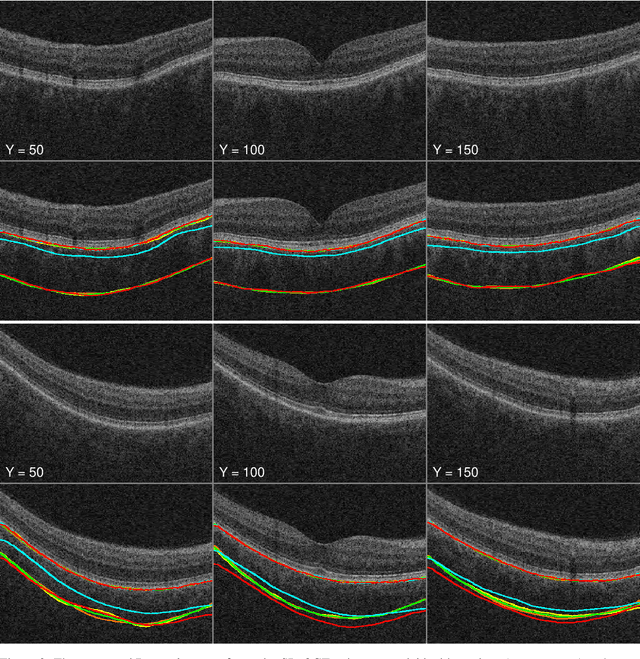
Abstract:Background: Changes in choroidal thickness are associated with various ocular diseases and the choroid can be imaged using spectral-domain optical coherence tomography (SDOCT) and enhanced depth imaging OCT (EDIOCT). New Method: Eighty macular SDOCT volumes from 80 patients were obtained using the Zeiss Cirrus machine. Eleven additional control subjects had two Cirrus scans done in one visit along with EDIOCT using the Heidelberg Spectralis machine. To automatically segment choroidal layers from the OCT volumes, our graph-theoretic approach was utilized. The segmentation results were compared with reference standards from two graders, and the accuracy of automated segmentation was calculated using unsigned to signed border positioning thickness errors and Dice similarity coefficient (DSC). The repeatability and reproducibility of our choroidal thicknesses were determined by intraclass correlation coefficient (ICC), coefficient of variation (CV), and repeatability coefficient (RC). Results: The mean unsigned to signed border positioning errors for the choroidal inner and outer surfaces are 3.39plusminus1.26microns (mean plusminus SD) to minus1.52 plusminus 1.63microns and 16.09 plusminus 6.21microns to 4.73 plusminus 9.53microns, respectively. The mean unsigned to signed choroidal thickness errors are 16.54 plusminus 6.47microns to 6.25 plusminus 9.91microns, and the mean DSC is 0.949 plusminus 0.025. The ICC (95% CI), CV, RC values are 0.991 (0.977 to 0.997), 2.48%, 3.15microns for the repeatability and 0.991 (0.977 to 0.997), 2.49%, 0.53microns for the reproducibility studies, respectively. Comparison with Existing Method(s): The proposed method outperformed our previous method using choroidal vessel segmentation and inter-grader variability. Conclusions: This automated segmentation method can reliably measure choroidal thickness using different OCT platforms.
Optimal Multiple Surface Segmentation with Convex Priors in Irregularly Sampled Space
May 16, 2017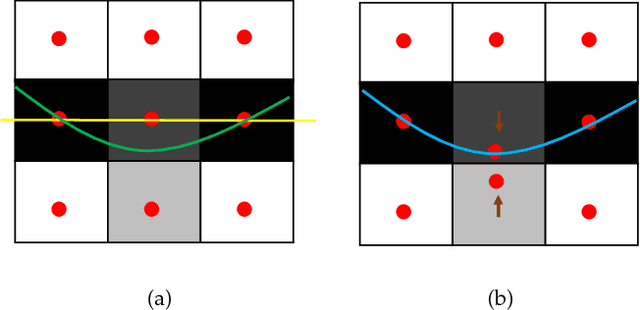
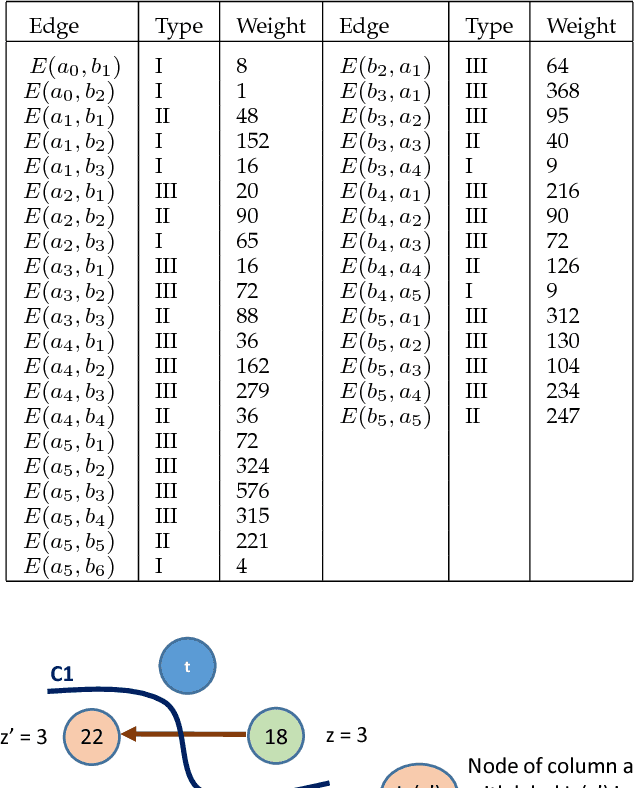
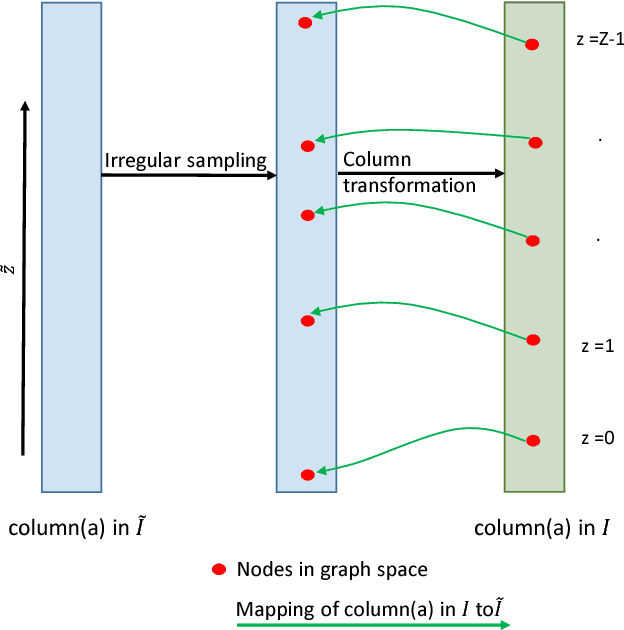
Abstract:Optimal surface segmentation is widely used in numerous medical image segmentation applications. However, nodes in the graph based optimal surface segmentation method typically encode uniformly distributed orthogonal voxels of the volume. Thus the segmentation cannot attain an accuracy greater than a single unit voxel, i.e. the distance between two adjoining nodes in graph space. Segmentation accuracy higher than a unit voxel is achievable by exploiting partial volume information in the voxels which shall result in non-equidistant spacing between adjoining graph nodes. This paper reports a generalized graph based optimal multiple surface segmentation method with convex priors which segments the target surfaces in irregularly sampled space. The proposed method allows non-equidistant spacing between the adjoining graph nodes to achieve subvoxel accurate segmentation by utilizing the partial volume information in the voxels. The partial volume information in the voxels is exploited by computing a displacement field from the original volume data to identify the subvoxel accurate centers within each voxel resulting in non-equidistant spacing between the adjoining graph nodes. The smoothness of each surface modelled as a convex constraint governs the connectivity and regularity of the surface. We employ an edge-based graph representation to incorporate the necessary constraints and the globally optimal solution is obtained by computing a minimum s-t cut. The proposed method was validated on 25 optical coherence tomography image volumes of the retina and 10 intravascular multi-frame ultrasound image datasets for subvoxel and super resolution segmentation accuracy. In all cases, the approach yielded highly accurate results. Our approach can be readily extended to higher-dimensional segmentations.
Thickness Mapping of Eleven Retinal Layers in Normal Eyes Using Spectral Domain Optical Coherence Tomography
Dec 11, 2013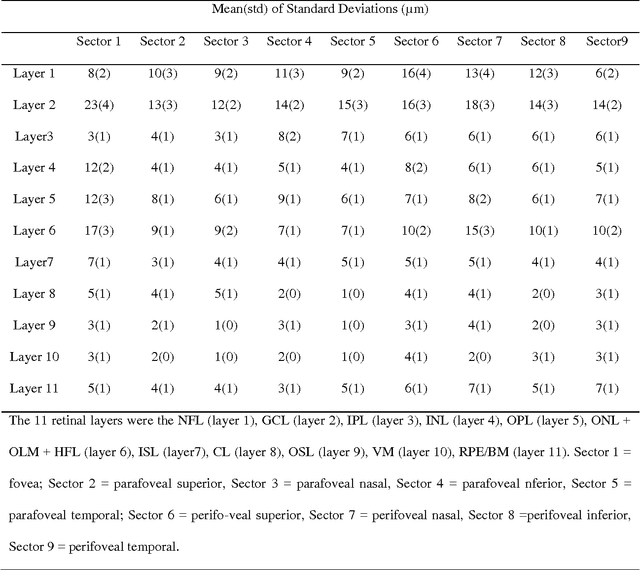
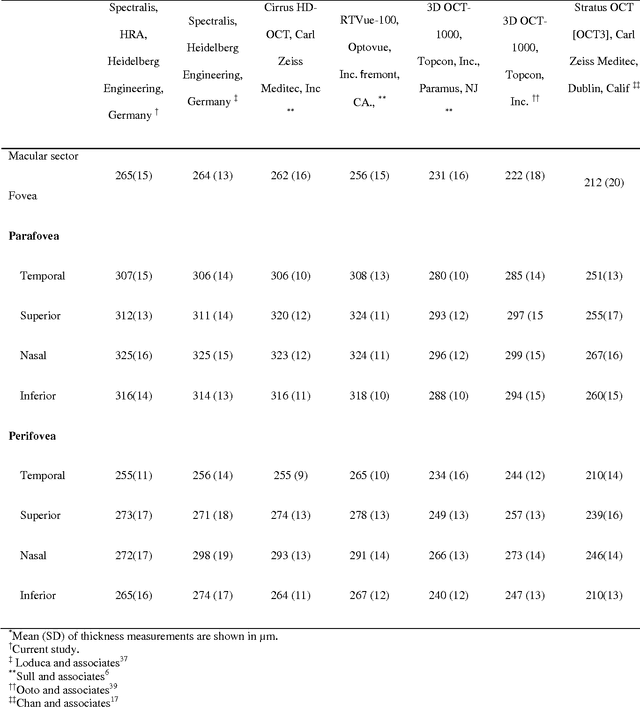
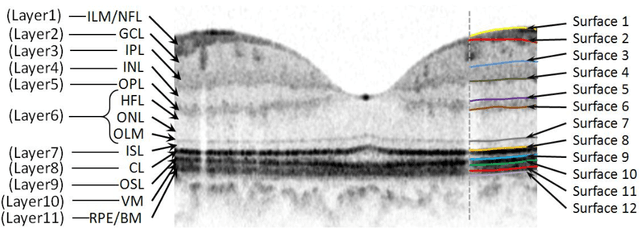
Abstract:Purpose. This study was conducted to determine the thickness map of eleven retinal layers in normal subjects by spectral domain optical coherence tomography (SD-OCT) and evaluate their association with sex and age. Methods. Mean regional retinal thickness of 11 retinal layers were obtained by automatic three-dimensional diffusion-map-based method in 112 normal eyes of 76 Iranian subjects. Results. The thickness map of central foveal area in layer 1, 3, and 4 displayed the minimum thickness (P<0.005 for all). Maximum thickness was observed in nasal to the fovea of layer 1 (P<0.001) and in a circular pattern in the parafoveal retinal area of layers 2, 3 and 4 and in central foveal area of layer 6 (P<0.001). Temporal and inferior quadrants of the total retinal thickness and most of other quadrants of layer 1 were significantly greater in the men than in the women. Surrounding eight sectors of total retinal thickness and a limited number of sectors in layer 1 and 4 significantly correlated with age. Conclusion. SD-OCT demonstrated the three-dimensional thickness distribution of retinal layers in normal eyes. Thickness of layers varied with sex and age and in different sectors. These variables should be considered while evaluating macular thickness.
Intra-Retinal Layer Segmentation of 3D Optical Coherence Tomography Using Coarse Grained Diffusion Map
Oct 08, 2012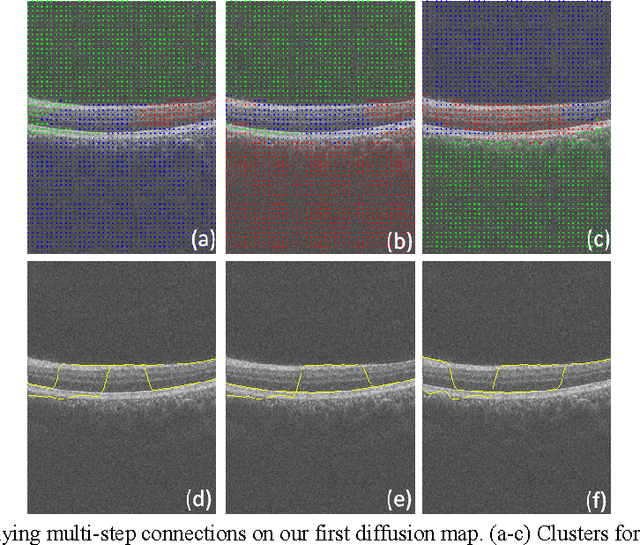
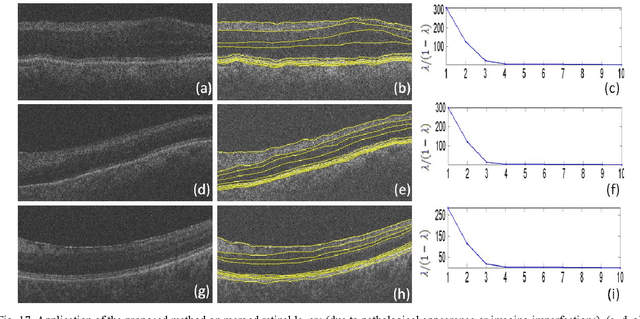
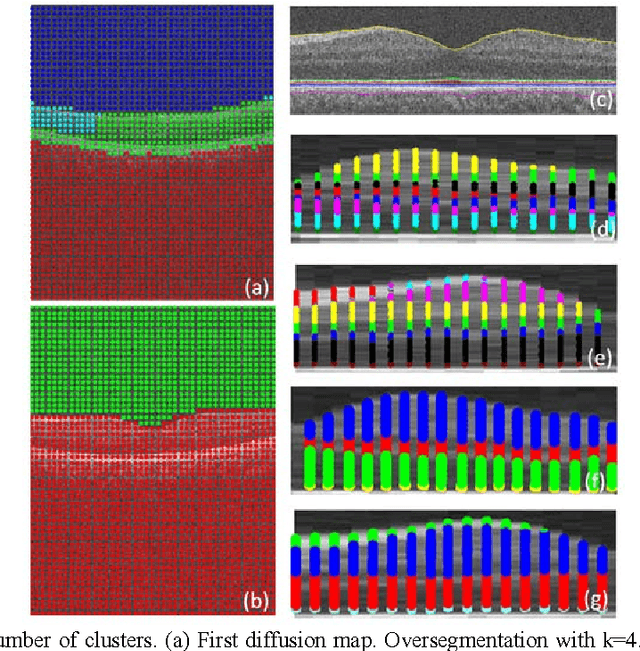

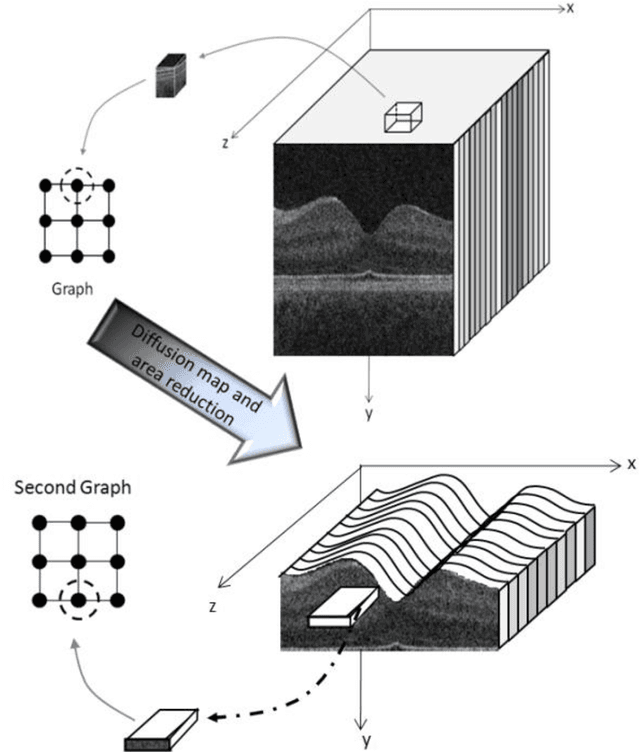
Abstract:Optical coherence tomography (OCT) is a powerful and noninvasive method for retinal imaging. In this paper, we introduce a fast segmentation method based on a new variant of spectral graph theory named diffusion maps. The research is performed on spectral domain (SD) OCT images depicting macular and optic nerve head appearance. The presented approach does not require edge-based image information and relies on regional image texture. Consequently, the proposed method demonstrates robustness in situations of low image contrast or poor layer-to-layer image gradients. Diffusion mapping is applied to 2D and 3D OCT datasets composed of two steps, one for partitioning the data into important and less important sections, and another one for localization of internal layers.In the first step, the pixels/voxels are grouped in rectangular/cubic sets to form a graph node.The weights of a graph are calculated based on geometric distances between pixels/voxels and differences of their mean intensity.The first diffusion map clusters the data into three parts, the second of which is the area of interest. The other two sections are eliminated from the remaining calculations. In the second step, the remaining area is subjected to another diffusion map assessment and the internal layers are localized based on their textural similarities.The proposed method was tested on 23 datasets from two patient groups (glaucoma and normals). The mean unsigned border positioning errors(mean - SD) was 8.52 - 3.13 and 7.56 - 2.95 micrometer for the 2D and 3D methods, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge