Andreas Wahle
FiAt-Net: Detecting Fibroatheroma Plaque Cap in 3D Intravascular OCT Images
Sep 13, 2024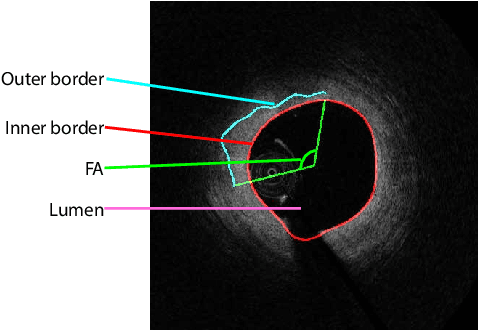
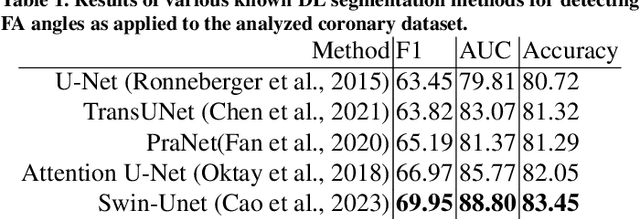
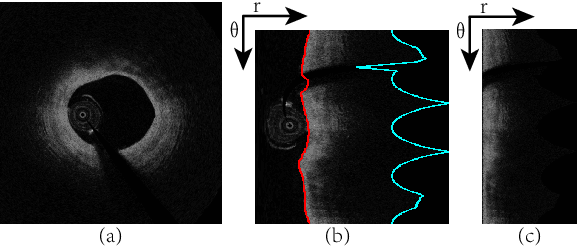
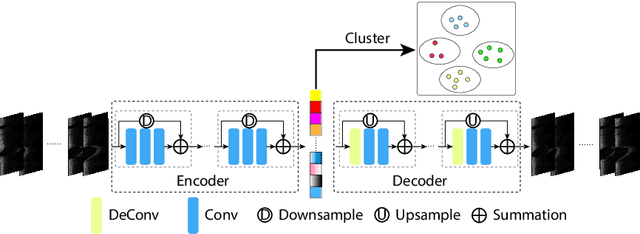
Abstract:The key manifestation of coronary artery disease (CAD) is development of fibroatheromatous plaque, the cap of which may rupture and subsequently lead to coronary artery blocking and heart attack. As such, quantitative analysis of coronary plaque, its plaque cap, and consequently the cap's likelihood to rupture are of critical importance when assessing a risk of cardiovascular events. This paper reports a new deep learning based approach, called FiAt-Net, for detecting angular extent of fibroatheroma (FA) and segmenting its cap in 3D intravascular optical coherence tomography (IVOCT) images. IVOCT 2D image frames are first associated with distinct clusters and data from each cluster are used for model training. As plaque is typically focal and thus unevenly distributed, a binary partitioning method is employed to identify FA plaque areas to focus on to mitigate the data imbalance issue. Additional image representations (called auxiliary images) are generated to capture IVOCT intensity changes to help distinguish FA and non-FA areas on the coronary wall. Information in varying scales is derived from the original IVOCT and auxiliary images, and a multi-head self-attention mechanism is employed to fuse such information. Our FiAt-Net achieved high performance on a 3D IVOCT coronary image dataset, demonstrating its effectiveness in accurately detecting FA cap in IVOCT images.
Automated segmentation of choroidal layers from 3-dimensional macular optical coherence tomography scans
Mar 11, 2021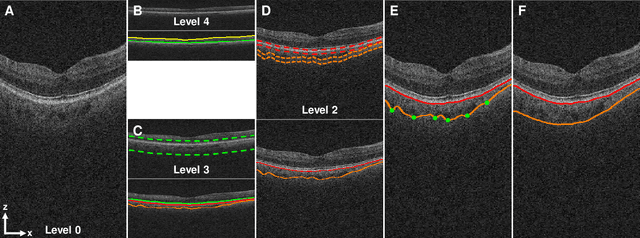
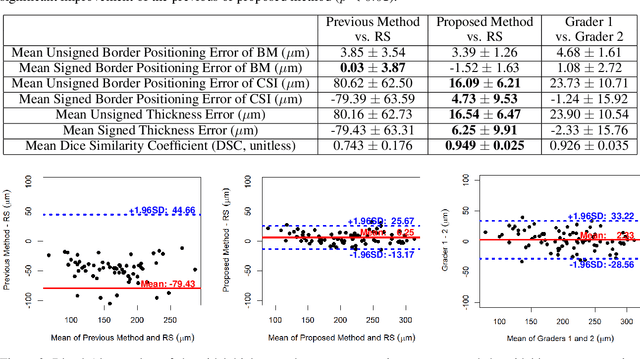
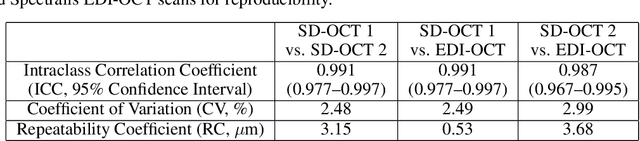
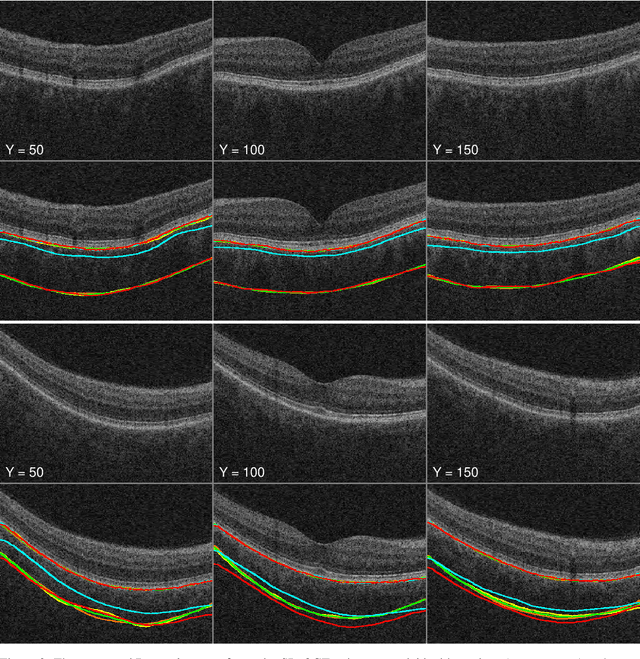
Abstract:Background: Changes in choroidal thickness are associated with various ocular diseases and the choroid can be imaged using spectral-domain optical coherence tomography (SDOCT) and enhanced depth imaging OCT (EDIOCT). New Method: Eighty macular SDOCT volumes from 80 patients were obtained using the Zeiss Cirrus machine. Eleven additional control subjects had two Cirrus scans done in one visit along with EDIOCT using the Heidelberg Spectralis machine. To automatically segment choroidal layers from the OCT volumes, our graph-theoretic approach was utilized. The segmentation results were compared with reference standards from two graders, and the accuracy of automated segmentation was calculated using unsigned to signed border positioning thickness errors and Dice similarity coefficient (DSC). The repeatability and reproducibility of our choroidal thicknesses were determined by intraclass correlation coefficient (ICC), coefficient of variation (CV), and repeatability coefficient (RC). Results: The mean unsigned to signed border positioning errors for the choroidal inner and outer surfaces are 3.39plusminus1.26microns (mean plusminus SD) to minus1.52 plusminus 1.63microns and 16.09 plusminus 6.21microns to 4.73 plusminus 9.53microns, respectively. The mean unsigned to signed choroidal thickness errors are 16.54 plusminus 6.47microns to 6.25 plusminus 9.91microns, and the mean DSC is 0.949 plusminus 0.025. The ICC (95% CI), CV, RC values are 0.991 (0.977 to 0.997), 2.48%, 3.15microns for the repeatability and 0.991 (0.977 to 0.997), 2.49%, 0.53microns for the reproducibility studies, respectively. Comparison with Existing Method(s): The proposed method outperformed our previous method using choroidal vessel segmentation and inter-grader variability. Conclusions: This automated segmentation method can reliably measure choroidal thickness using different OCT platforms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge