Matti Kiupel
Deep Learning model predicts the c-Kit-11 mutational status of canine cutaneous mast cell tumors by HE stained histological slides
Jan 02, 2024
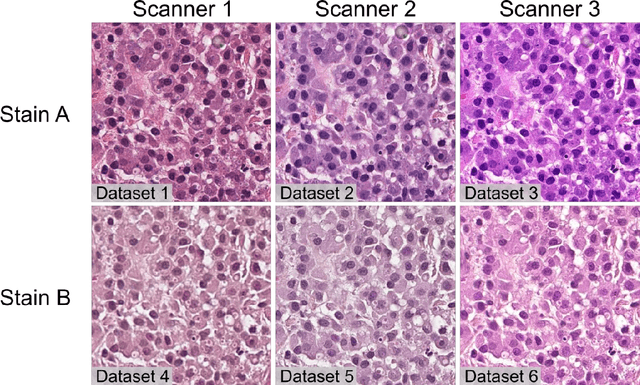
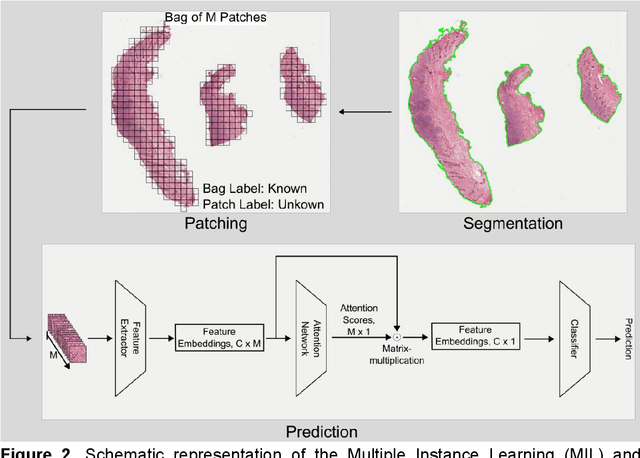
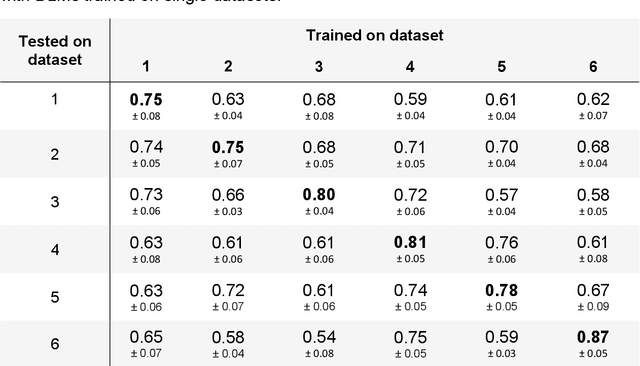
Abstract:Numerous prognostic factors are currently assessed histopathologically in biopsies of canine mast cell tumors to evaluate clinical behavior. In addition, PCR analysis of the c-Kit exon 11 mutational status is often performed to evaluate the potential success of a tyrosine kinase inhibitor therapy. This project aimed at training deep learning models (DLMs) to identify the c-Kit-11 mutational status of MCTs solely based on morphology without additional molecular analysis. HE slides of 195 mutated and 173 non-mutated tumors were stained consecutively in two different laboratories and scanned with three different slide scanners. This resulted in six different datasets (stain-scanner variations) of whole slide images. DLMs were trained with single and mixed datasets and their performances was assessed under scanner and staining domain shifts. The DLMs correctly classified HE slides according to their c-Kit 11 mutation status in, on average, 87% of cases for the best-suited stain-scanner variant. A relevant performance drop could be observed when the stain-scanner combination of the training and test dataset differed. Multi-variant datasets improved the average accuracy but did not reach the maximum accuracy of algorithms trained and tested on the same stain-scanner variant. In summary, DLM-assisted morphological examination of MCTs can predict c-Kit-exon 11 mutational status of MCTs with high accuracy. However, the recognition performance is impeded by a change of scanner or staining protocol. Larger data sets with higher numbers of scans originating from different laboratories and scanners may lead to more robust DLMs to identify c-Kit mutations in HE slides.
Nuclear Morphometry using a Deep Learning-based Algorithm has Prognostic Relevance for Canine Cutaneous Mast Cell Tumors
Sep 26, 2023Abstract:Variation in nuclear size and shape is an important criterion of malignancy for many tumor types; however, categorical estimates by pathologists have poor reproducibility. Measurements of nuclear characteristics (morphometry) can improve reproducibility, but manual methods are time consuming. In this study, we evaluated fully automated morphometry using a deep learning-based algorithm in 96 canine cutaneous mast cell tumors with information on patient survival. Algorithmic morphometry was compared with karyomegaly estimates by 11 pathologists, manual nuclear morphometry of 12 cells by 9 pathologists, and the mitotic count as a benchmark. The prognostic value of automated morphometry was high with an area under the ROC curve regarding the tumor-specific survival of 0.943 (95% CI: 0.889 - 0.996) for the standard deviation (SD) of nuclear area, which was higher than manual morphometry of all pathologists combined (0.868, 95% CI: 0.737 - 0.991) and the mitotic count (0.885, 95% CI: 0.765 - 1.00). At the proposed thresholds, the hazard ratio for algorithmic morphometry (SD of nuclear area $\geq 9.0 \mu m^2$) was 18.3 (95% CI: 5.0 - 67.1), for manual morphometry (SD of nuclear area $\geq 10.9 \mu m^2$) 9.0 (95% CI: 6.0 - 13.4), for karyomegaly estimates 7.6 (95% CI: 5.7 - 10.1), and for the mitotic count 30.5 (95% CI: 7.8 - 118.0). Inter-rater reproducibility for karyomegaly estimates was fair ($\kappa$ = 0.226) with highly variable sensitivity/specificity values for the individual pathologists. Reproducibility for manual morphometry (SD of nuclear area) was good (ICC = 0.654). This study supports the use of algorithmic morphometry as a prognostic test to overcome the limitations of estimates and manual measurements.
Deep Learning-Based Automatic Assessment of AgNOR-scores in Histopathology Images
Dec 15, 2022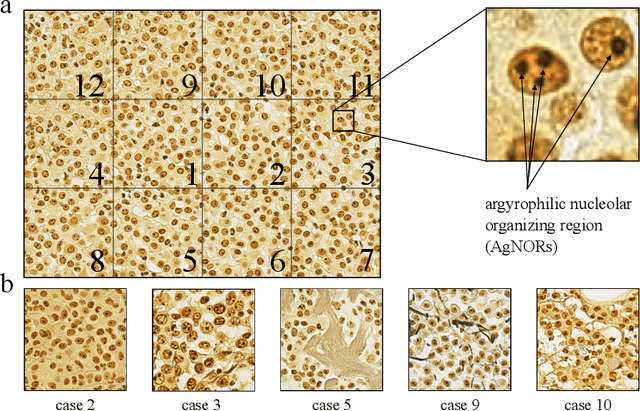
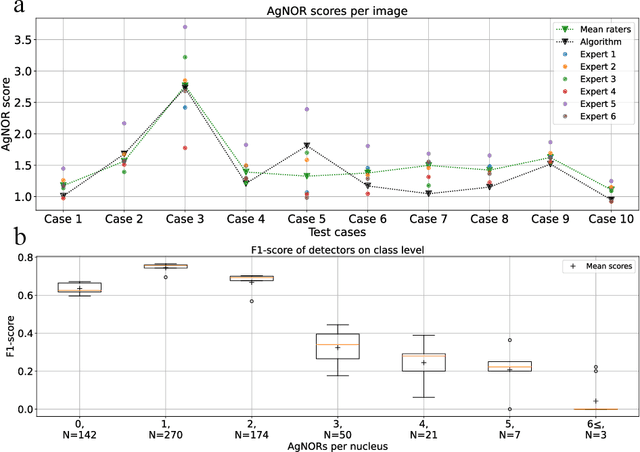
Abstract:Nucleolar organizer regions (NORs) are parts of the DNA that are involved in RNA transcription. Due to the silver affinity of associated proteins, argyrophilic NORs (AgNORs) can be visualized using silver-based staining. The average number of AgNORs per nucleus has been shown to be a prognostic factor for predicting the outcome of many tumors. Since manual detection of AgNORs is laborious, automation is of high interest. We present a deep learning-based pipeline for automatically determining the AgNOR-score from histopathological sections. An additional annotation experiment was conducted with six pathologists to provide an independent performance evaluation of our approach. Across all raters and images, we found a mean squared error of 0.054 between the AgNOR- scores of the experts and those of the model, indicating that our approach offers performance comparable to humans.
How Many Annotators Do We Need? -- A Study on the Influence of Inter-Observer Variability on the Reliability of Automatic Mitotic Figure Assessment
Jan 08, 2021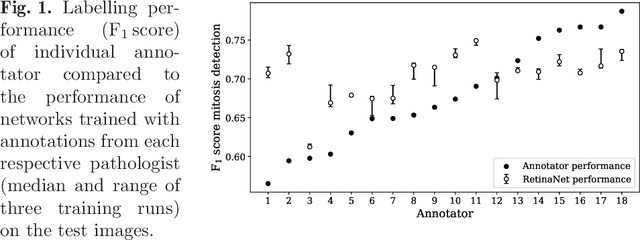
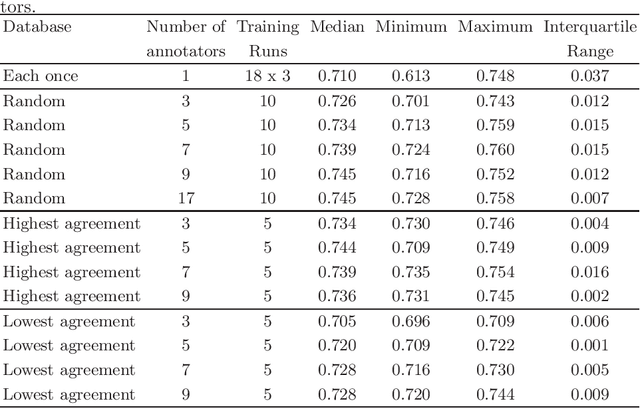
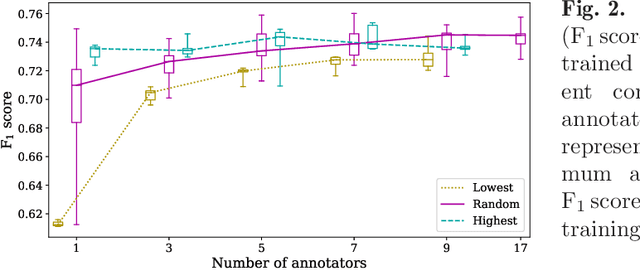
Abstract:Density of mitotic figures in histologic sections is a prognostically relevant characteristic for many tumours. Due to high inter-pathologist variability, deep learning-based algorithms are a promising solution to improve tumour prognostication. Pathologists are the gold standard for database development, however, labelling errors may hamper development of accurate algorithms. In the present work we evaluated the benefit of multi-expert consensus (n = 3, 5, 7, 9, 11) on algorithmic performance. While training with individual databases resulted in highly variable F$_1$ scores, performance was notably increased and more consistent when using the consensus of three annotators. Adding more annotators only resulted in minor improvements. We conclude that databases by few pathologists and high label accuracy may be the best compromise between high algorithmic performance and time investment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge