Matthias Stuber
1 and 2, §
ALMA: a mathematics-driven approach for determining tuning parameters in generalized LASSO problems, with applications to MRI
Jun 27, 2024Abstract:Magnetic Resonance Imaging (MRI) is a powerful technique employed for non-invasive in vivo visualization of internal structures. Sparsity is often deployed to accelerate the signal acquisition or overcome the presence of motion artifacts, improving the quality of image reconstruction. Image reconstruction algorithms use TV-regularized LASSO (Total Variation-regularized LASSO) to retrieve the missing information of undersampled signals, by cleaning the data of noise and while optimizing sparsity. A tuning parameter moderates the balance between these two aspects; its choice affecting the quality of the reconstructions. Currently, there is a lack of general deterministic techniques to choose these parameters, which are oftentimes manually selected and thus hinder the reliability of the reconstructions. Here, we present ALMA (Algorithm for Lagrange Multipliers Approximation), an iterative mathematics-inspired technique that computes tuning parameters for generalized LASSO problems during MRI reconstruction. We analyze quantitatively the performance of these parameters for imaging reconstructions via TV-LASSO in an MRI context on phantoms. Although our study concentrates on TV-LASSO, the techniques developed here hold significant promise for a wide array of applications. ALMA is not only adaptable to more generalized LASSO problems but is also robust to accommodate other forms of regularization beyond total variation. Moreover, it extends effectively to handle non-Cartesian sampling trajectories, broadening its utility in complex data reconstruction scenarios. More generally, ALMA provides a powerful tool for numerically solving constrained optimization problems across various disciplines, offering a versatile and impactful solution for advanced computational challenges.
FaBiAN: A Fetal Brain magnetic resonance Acquisition Numerical phantom
Sep 06, 2021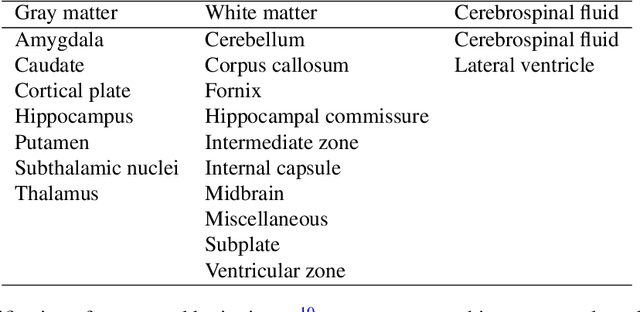
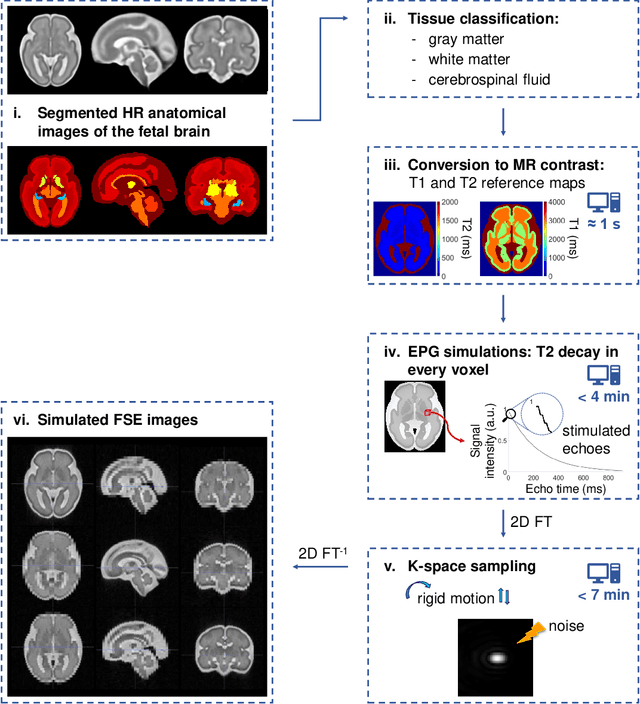
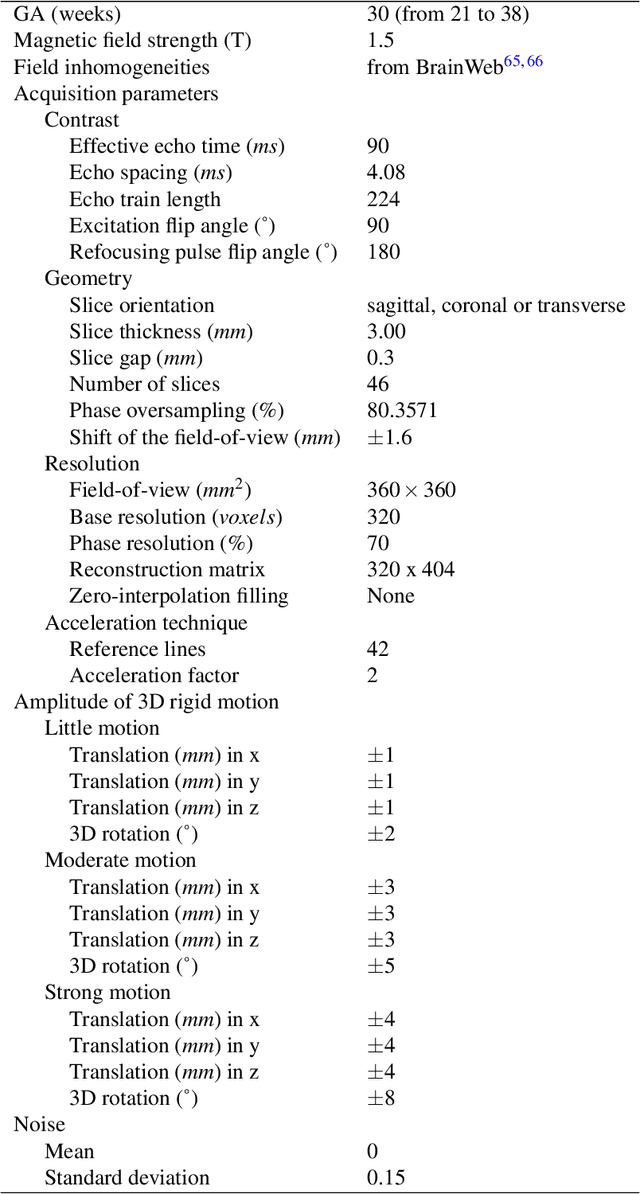
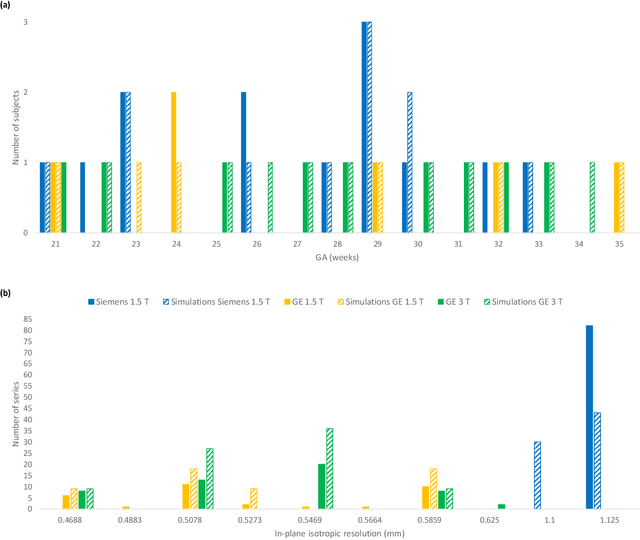
Abstract:Accurate characterization of in utero human brain maturation is critical as it involves complex and interconnected structural and functional processes that may influence health later in life. Magnetic resonance imaging is a powerful tool to investigate equivocal neurological patterns during fetal development. However, the number of acquisitions of satisfactory quality available in this cohort of sensitive subjects remains scarce, thus hindering the validation of advanced image processing techniques. Numerical phantoms can mitigate these limitations by providing a controlled environment with a known ground truth. In this work, we present FaBiAN, an open-source Fetal Brain magnetic resonance Acquisition Numerical phantom that simulates clinical T2-weighted fast spin echo sequences of the fetal brain. This unique tool is based on a general, flexible and realistic setup that includes stochastic fetal movements, thus providing images of the fetal brain throughout maturation comparable to clinical acquisitions. We demonstrate its value to evaluate the robustness and optimize the accuracy of an algorithm for super-resolution fetal brain magnetic resonance imaging from simulated motion-corrupted 2D low-resolution series as compared to a synthetic high-resolution reference volume. We also show that the images generated can complement clinical datasets to support data-intensive deep learning methods for fetal brain tissue segmentation.
Free-running SIMilarity-Based Angiography (SIMBA) for simplified anatomical MR imaging of the heart
Jul 13, 2020



Abstract:Purpose: Whole-heart MRA techniques typically target pre-determined motion states and address cardiac and respiratory dynamics independently. We propose a novel fast reconstruction algorithm, applicable to ungated free-running sequences, that leverages inherent similarities in the acquired data to avoid such physiological constraints. Theory and Methods: The proposed SIMilarity-Based Angiography (SIMBA) method clusters the continuously acquired k-space data in order to find a motion-consistent subset that can be reconstructed into a motion-suppressed whole-heart MRA. Free-running 3D radial datasets from six ferumoxytol-enhanced scans of pediatric cardiac patients and twelve non-contrast scans of healthy volunteers were reconstructed with a non-motion-suppressed regridding of all the acquired data (All Data), our proposed SIMBA method, and a previously published free-running framework (FRF) that uses cardiac and respiratory self-gating and compressed sensing. Images were compared for blood-myocardium interface sharpness, contrast ratio, and visibility of coronary artery ostia. Results: Both the fast SIMBA reconstruction (~20s) and the FRF provided significantly higher blood-myocardium sharpness than All Data (P<0.001). No significant difference was observed among the former two. Significantly higher blood-myocardium contrast ratio was obtained with SIMBA compared to All Data and FRF (P<0.01). More coronary ostia could be visualized with both SIMBA and FRF than with All Data (All Data: 4/36, SIMBA: 30/36, FRF: 33/36, both P<0.001) but no significant difference was found between the first two. Conclusion: The combination of free-running sequences and the fast SIMBA reconstruction, which operates without a priori assumptions related to physiological motion, forms a simple workflow for obtaining whole-heart MRA with sharp anatomical structures.
Time-Dependent Deep Image Prior for Dynamic MRI
Oct 03, 2019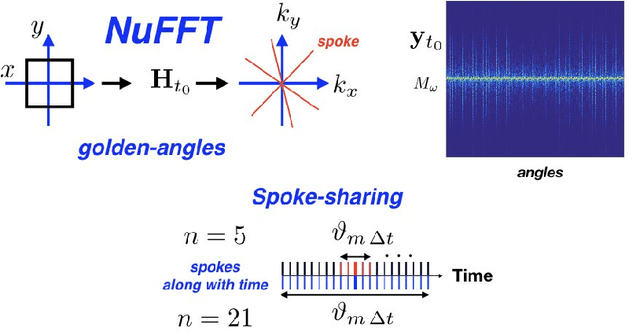
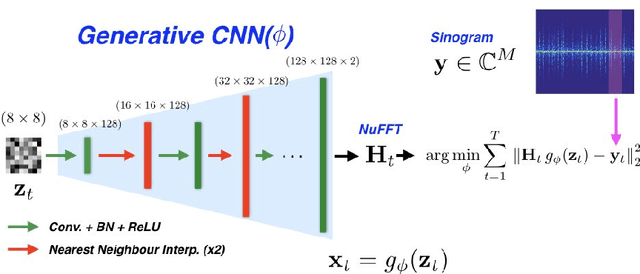
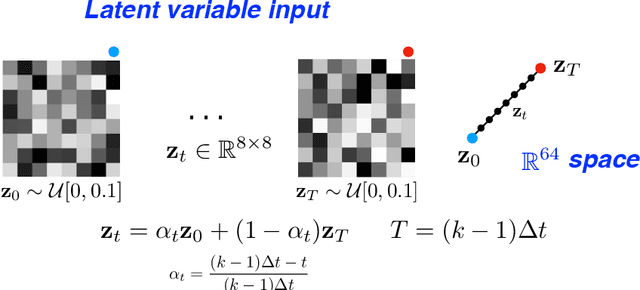
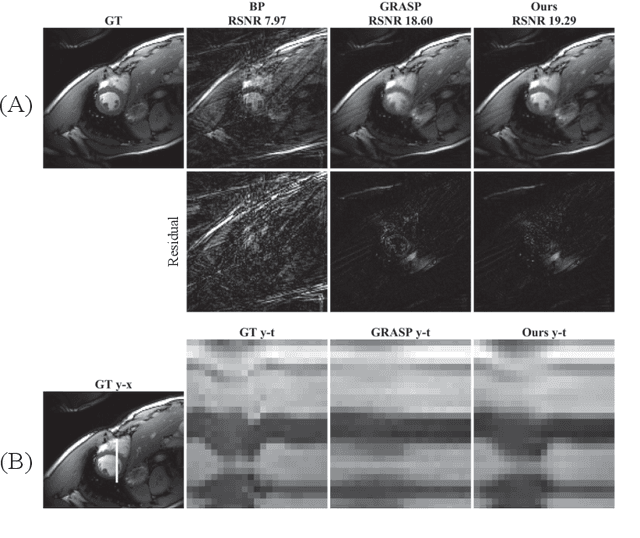
Abstract:We propose a novel unsupervised deep-learning-based algorithm to solve the inverse problem found in dynamic magnetic resonance imaging (MRI). Our method needs neither prior training nor additional data; in particular, it does not require either electrocardiogram or spokes-reordering in the context of cardiac images. It generalizes to sequences of images the recently introduced deep-image-prior approach. The essence of the proposed algorithm is to proceed in two steps to fit k-space synthetic measurements to sparsely acquired dynamic MRI data. In the first step, we deploy a convolutional neural network (CNN) driven by a sequence of low-dimensional latent variables to generate a dynamic series of MRI images. In the second step, we submit the generated images to a nonuniform fast Fourier transform that represents the forward model of the MRI system. By manipulating the weights of the CNN, we fit our synthetic measurements to the acquired MRI data. The corresponding images from the CNN then provide the output of our system; their evolution through time is driven by controlling the sequence of latent variables whose interpolation gives access to the sub-frame---or even continuous---temporal control of reconstructed dynamic images. We perform experiments on simulated and real cardiac images of a fetus acquired through 5-spoke-based golden-angle measurements. Our results show improvement over the current state-of-the-art.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge