Masoud Zarepisheh
Automating High Quality RT Planning at Scale
Jan 21, 2025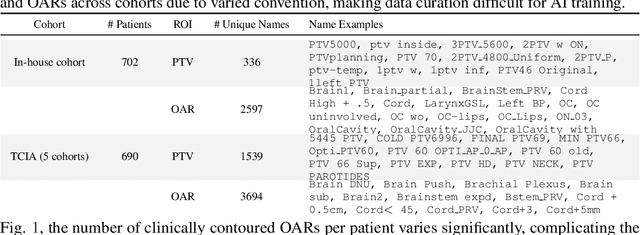
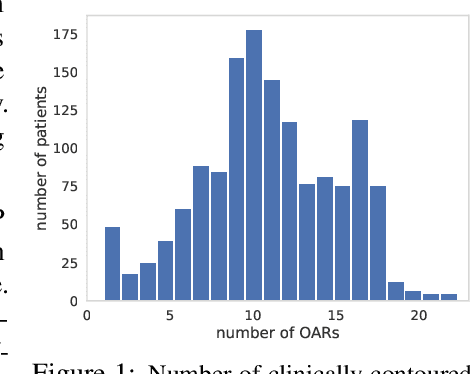
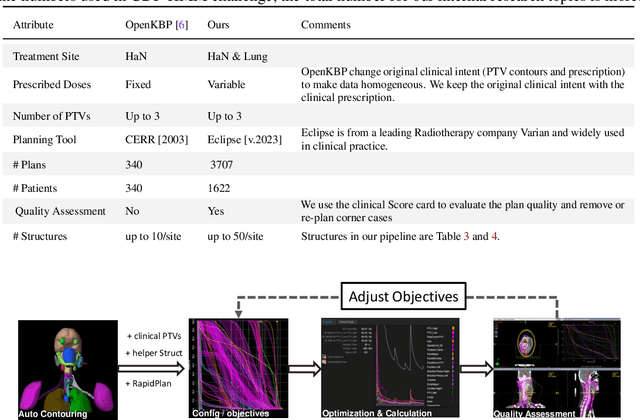
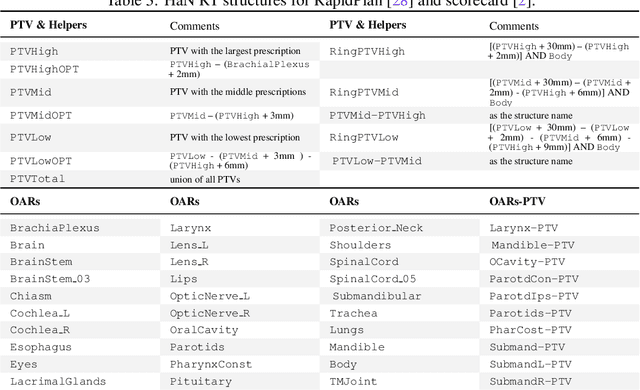
Abstract:Radiotherapy (RT) planning is complex, subjective, and time-intensive. Advances in artificial intelligence (AI) promise to improve its precision, efficiency, and consistency, but progress is often limited by the scarcity of large, standardized datasets. To address this, we introduce the Automated Iterative RT Planning (AIRTP) system, a scalable solution for generating high-quality treatment plans. This scalable solution is designed to generate substantial volumes of consistently high-quality treatment plans, overcoming a key obstacle in the advancement of AI-driven RT planning. Our AIRTP pipeline adheres to clinical guidelines and automates essential steps, including organ-at-risk (OAR) contouring, helper structure creation, beam setup, optimization, and plan quality improvement, using AI integrated with RT planning software like Eclipse of Varian. Furthermore, a novel approach for determining optimization parameters to reproduce 3D dose distributions, i.e. a method to convert dose predictions to deliverable treatment plans constrained by machine limitations. A comparative analysis of plan quality reveals that our automated pipeline produces treatment plans of quality comparable to those generated manually, which traditionally require several hours of labor per plan. Committed to public research, the first data release of our AIRTP pipeline includes nine cohorts covering head-and-neck and lung cancer sites to support an AAPM 2025 challenge. This data set features more than 10 times the number of plans compared to the largest existing well-curated public data set to our best knowledge. Repo:{https://github.com/RiqiangGao/GDP-HMM_AAPMChallenge}
RMSim: Controlled Respiratory Motion Simulation on Static Patient Scans
Jan 26, 2023Abstract:This work aims to generate realistic anatomical deformations from static patient scans. Specifically, we present a method to generate these deformations/augmentations via deep learning driven respiratory motion simulation that provides the ground truth for validating deformable image registration (DIR) algorithms and driving more accurate deep learning based DIR. We present a novel 3D Seq2Seq deep learning respiratory motion simulator (RMSim) that learns from 4D-CT images and predicts future breathing phases given a static CT image. The predicted respiratory patterns, represented by time-varying displacement vector fields (DVFs) at different breathing phases, are modulated through auxiliary inputs of 1D breathing traces so that a larger amplitude in the trace results in more significant predicted deformation. Stacked 3D-ConvLSTMs are used to capture the spatial-temporal respiration patterns. Training loss includes a smoothness loss in the DVF and mean-squared error between the predicted and ground truth phase images. A spatial transformer deforms the static CT with the predicted DVF to generate the predicted phase image. 10-phase 4D-CTs of 140 internal patients were used to train and test RMSim. The trained RMSim was then used to augment a public DIR challenge dataset for training VoxelMorph to show the effectiveness of RMSim-generated deformation augmentation. We validated our RMSim output with both private and public benchmark datasets (healthy and cancer patients). The proposed approach can be used for validating DIR algorithms as well as for patient-specific augmentations to improve deep learning DIR algorithms. The code, pretrained models, and augmented DIR validation datasets will be released at https://github.com/nadeemlab/SeqX2Y.
Domain Knowledge Driven 3D Dose Prediction Using Moment-Based Loss Function
Jul 07, 2022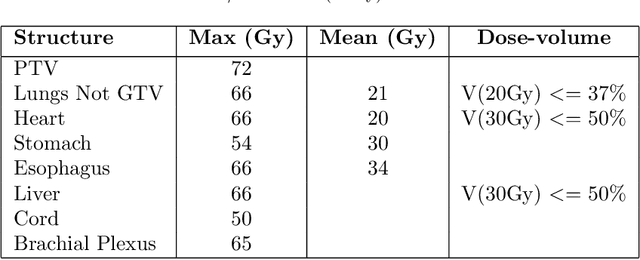
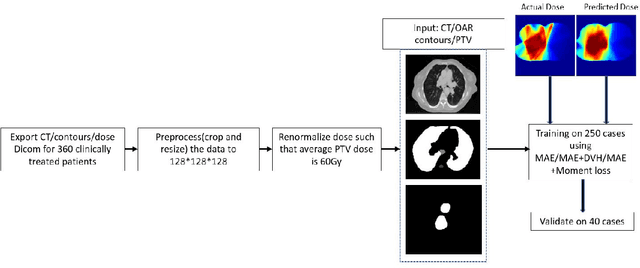
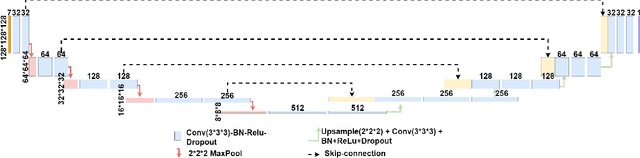
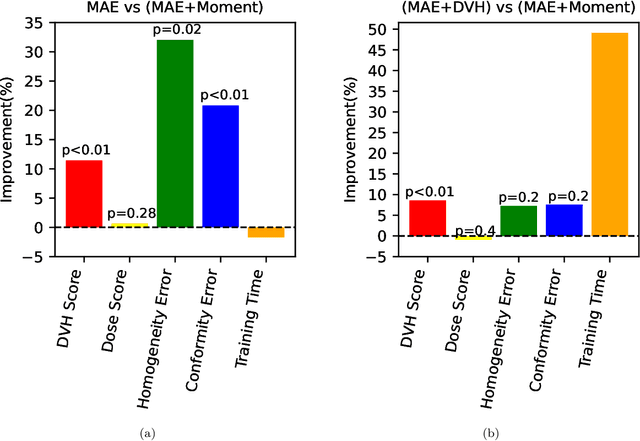
Abstract:Dose volume histogram (DVH) metrics are widely accepted evaluation criteria in the clinic. However, incorporating these metrics into deep learning dose prediction models is challenging due to their non-convexity and non-differentiability. We propose a novel moment-based loss function for predicting 3D dose distribution for the challenging conventional lung intensity modulated radiation therapy (IMRT) plans. The moment-based loss function is convex and differentiable and can easily incorporate DVH metrics in any deep learning framework without computational overhead. The moments can also be customized to reflect the clinical priorities in 3D dose prediction. For instance, using high-order moments allows better prediction in high-dose areas for serial structures. We used a large dataset of 360 (240 for training, 50 for validation and 70 for testing) conventional lung patients with 2Gy $\times$ 30 fractions to train the deep learning (DL) model using clinically treated plans at our institution. We trained a UNet like CNN architecture using computed tomography (CT), planning target volume (PTV) and organ-at-risk contours (OAR) as input to infer corresponding voxel-wise 3D dose distribution. We evaluated three different loss functions: (1) The popular Mean Absolute Error (MAE) Loss, (2) the recently developed MAE + DVH Loss, and (3) the proposed MAE + Moments Loss. The quality of the predictions was compared using different DVH metrics as well as dose-score and DVH-score, recently introduced by the AAPM knowledge-based planning grand challenge. Model with (MAE + Moment) loss function outperformed the model with MAE loss by significantly improving the DVH-score (11%, p$<$0.01) while having similar computational cost. It also outperformed the model trained with (MAE+DVH) by significantly improving the computational cost (48%) and the DVH-score (8%, p$<$0.01).
Deep Learning 3D Dose Prediction for Conventional Lung IMRT Using Consistent/Unbiased Automated Plans
Jun 07, 2021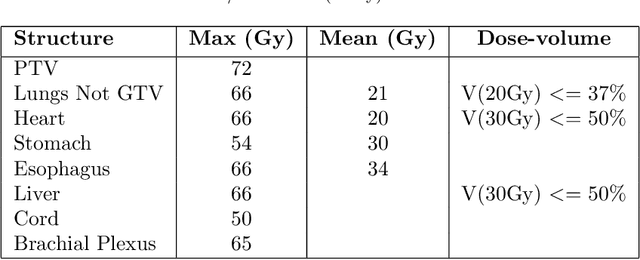
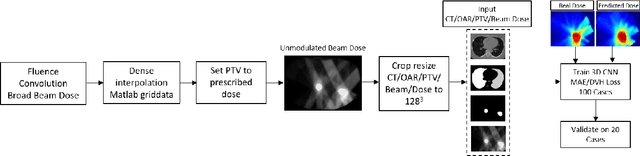
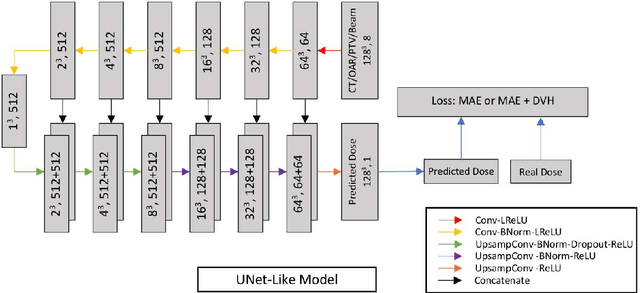

Abstract:Deep learning (DL) 3D dose prediction has recently gained a lot of attention. However, the variability of plan quality in the training dataset, generated manually by planners with wide range of expertise, can dramatically effect the quality of the final predictions. Moreover, any changes in the clinical criteria requires a new set of manually generated plans by planners to build a new prediction model. In this work, we instead use consistent plans generated by our in-house automated planning system (named ``ECHO'') to train the DL model. ECHO (expedited constrained hierarchical optimization) generates consistent/unbiased plans by solving large-scale constrained optimization problems sequentially. If the clinical criteria changes, a new training data set can be easily generated offline using ECHO, with no or limited human intervention, making the DL-based prediction model easily adaptable to the changes in the clinical practice. We used 120 conventional lung patients (100 for training, 20 for testing) with different beam configurations and trained our DL-model using manually-generated as well as automated ECHO plans. We evaluated different inputs: (1) CT+(PTV/OAR)contours, and (2) CT+contours+beam configurations, and different loss functions: (1) MAE (mean absolute error), and (2) MAE+DVH (dose volume histograms). The quality of the predictions was compared using different DVH metrics as well as dose-score and DVH-score, recently introduced by the AAPM knowledge-based planning grand challenge. The best results were obtained using automated ECHO plans and CT+contours+beam as training inputs and MAE+DVH as loss function.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge