Parmida Ghahremani
An AI-Ready Multiplex Staining Dataset for Reproducible and Accurate Characterization of Tumor Immune Microenvironment
May 25, 2023



Abstract:We introduce a new AI-ready computational pathology dataset containing restained and co-registered digitized images from eight head-and-neck squamous cell carcinoma patients. Specifically, the same tumor sections were stained with the expensive multiplex immunofluorescence (mIF) assay first and then restained with cheaper multiplex immunohistochemistry (mIHC). This is a first public dataset that demonstrates the equivalence of these two staining methods which in turn allows several use cases; due to the equivalence, our cheaper mIHC staining protocol can offset the need for expensive mIF staining/scanning which requires highly-skilled lab technicians. As opposed to subjective and error-prone immune cell annotations from individual pathologists (disagreement > 50%) to drive SOTA deep learning approaches, this dataset provides objective immune and tumor cell annotations via mIF/mIHC restaining for more reproducible and accurate characterization of tumor immune microenvironment (e.g. for immunotherapy). We demonstrate the effectiveness of this dataset in three use cases: (1) IHC quantification of CD3/CD8 tumor-infiltrating lymphocytes via style transfer, (2) virtual translation of cheap mIHC stains to more expensive mIF stains, and (3) virtual tumor/immune cellular phenotyping on standard hematoxylin images. The dataset is available at \url{https://github.com/nadeemlab/DeepLIIF}.
Domain Knowledge Driven 3D Dose Prediction Using Moment-Based Loss Function
Jul 07, 2022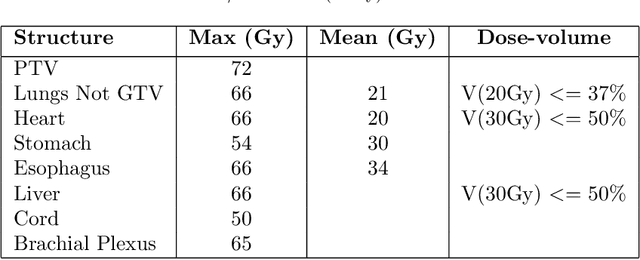
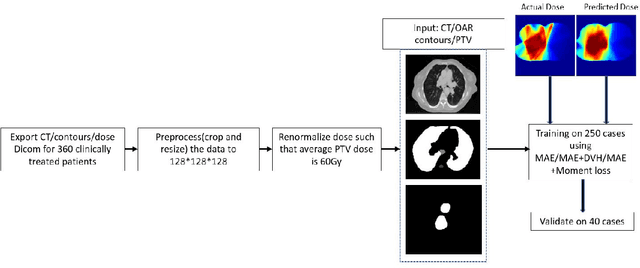
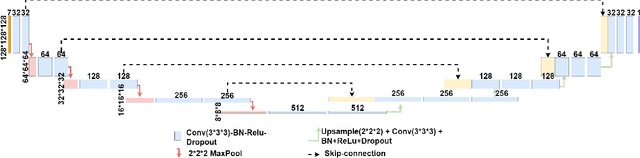
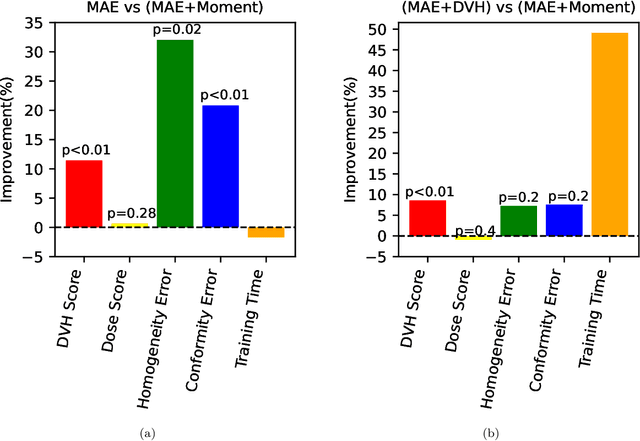
Abstract:Dose volume histogram (DVH) metrics are widely accepted evaluation criteria in the clinic. However, incorporating these metrics into deep learning dose prediction models is challenging due to their non-convexity and non-differentiability. We propose a novel moment-based loss function for predicting 3D dose distribution for the challenging conventional lung intensity modulated radiation therapy (IMRT) plans. The moment-based loss function is convex and differentiable and can easily incorporate DVH metrics in any deep learning framework without computational overhead. The moments can also be customized to reflect the clinical priorities in 3D dose prediction. For instance, using high-order moments allows better prediction in high-dose areas for serial structures. We used a large dataset of 360 (240 for training, 50 for validation and 70 for testing) conventional lung patients with 2Gy $\times$ 30 fractions to train the deep learning (DL) model using clinically treated plans at our institution. We trained a UNet like CNN architecture using computed tomography (CT), planning target volume (PTV) and organ-at-risk contours (OAR) as input to infer corresponding voxel-wise 3D dose distribution. We evaluated three different loss functions: (1) The popular Mean Absolute Error (MAE) Loss, (2) the recently developed MAE + DVH Loss, and (3) the proposed MAE + Moments Loss. The quality of the predictions was compared using different DVH metrics as well as dose-score and DVH-score, recently introduced by the AAPM knowledge-based planning grand challenge. Model with (MAE + Moment) loss function outperformed the model with MAE loss by significantly improving the DVH-score (11%, p$<$0.01) while having similar computational cost. It also outperformed the model trained with (MAE+DVH) by significantly improving the computational cost (48%) and the DVH-score (8%, p$<$0.01).
DeepLIIF: An Online Platform for Quantification of Clinical Pathology Slides
Apr 09, 2022



Abstract:In the clinic, resected tissue samples are stained with Hematoxylin-and-Eosin (H&E) and/or Immunhistochemistry (IHC) stains and presented to the pathologists on glass slides or as digital scans for diagnosis and assessment of disease progression. Cell-level quantification, e.g. in IHC protein expression scoring, can be extremely inefficient and subjective. We present DeepLIIF (https://deepliif.org), a first free online platform for efficient and reproducible IHC scoring. DeepLIIF outperforms current state-of-the-art approaches (relying on manual error-prone annotations) by virtually restaining clinical IHC slides with more informative multiplex immunofluorescence staining. Our DeepLIIF cloud-native platform supports (1) more than 150 proprietary/non-proprietary input formats via the Bio-Formats standard, (2) interactive adjustment, visualization, and downloading of the IHC quantification results and the accompanying restained images, (3) consumption of an exposed workflow API programmatically or through interactive plugins for open source whole slide image viewers such as QuPath/ImageJ, and (4) auto scaling to efficiently scale GPU resources based on user demand.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge