Joyce H. Keyak
PF-DAformer: Proximal Femur Segmentation via Domain Adaptive Transformer for Dual-Center QCT
Oct 30, 2025Abstract:Quantitative computed tomography (QCT) plays a crucial role in assessing bone strength and fracture risk by enabling volumetric analysis of bone density distribution in the proximal femur. However, deploying automated segmentation models in practice remains difficult because deep networks trained on one dataset often fail when applied to another. This failure stems from domain shift, where scanners, reconstruction settings, and patient demographics vary across institutions, leading to unstable predictions and unreliable quantitative metrics. Overcoming this barrier is essential for multi-center osteoporosis research and for ensuring that radiomics and structural finite element analysis results remain reproducible across sites. In this work, we developed a domain-adaptive transformer segmentation framework tailored for multi-institutional QCT. Our model is trained and validated on one of the largest hip fracture related research cohorts to date, comprising 1,024 QCT images scans from Tulane University and 384 scans from Rochester, Minnesota for proximal femur segmentation. To address domain shift, we integrate two complementary strategies within a 3D TransUNet backbone: adversarial alignment via Gradient Reversal Layer (GRL), which discourages the network from encoding site-specific cues, and statistical alignment via Maximum Mean Discrepancy (MMD), which explicitly reduces distributional mismatches between institutions. This dual mechanism balances invariance and fine-grained alignment, enabling scanner-agnostic feature learning while preserving anatomical detail.
ICGM-FRAX: Iterative Cross Graph Matching for Hip Fracture Risk Assessment using Dual-energy X-ray Absorptiometry Images
Apr 21, 2025Abstract:Hip fractures represent a major health concern, particularly among the elderly, often leading decreased mobility and increased mortality. Early and accurate detection of at risk individuals is crucial for effective intervention. In this study, we propose Iterative Cross Graph Matching for Hip Fracture Risk Assessment (ICGM-FRAX), a novel approach for predicting hip fractures using Dual-energy X-ray Absorptiometry (DXA) images. ICGM-FRAX involves iteratively comparing a test (subject) graph with multiple template graphs representing the characteristics of hip fracture subjects to assess the similarity and accurately to predict hip fracture risk. These graphs are obtained as follows. The DXA images are separated into multiple regions of interest (RoIs), such as the femoral head, shaft, and lesser trochanter. Radiomic features are then calculated for each RoI, with the central coordinates used as nodes in a graph. The connectivity between nodes is established according to the Euclidean distance between these coordinates. This process transforms each DXA image into a graph, where each node represents a RoI, and edges derived by the centroids of RoIs capture the spatial relationships between them. If the test graph closely matches a set of template graphs representing subjects with incident hip fractures, it is classified as indicating high hip fracture risk. We evaluated our method using 547 subjects from the UK Biobank dataset, and experimental results show that ICGM-FRAX achieved a sensitivity of 0.9869, demonstrating high accuracy in predicting hip fractures.
A Staged Approach using Machine Learning and Uncertainty Quantification to Predict the Risk of Hip Fracture
May 30, 2024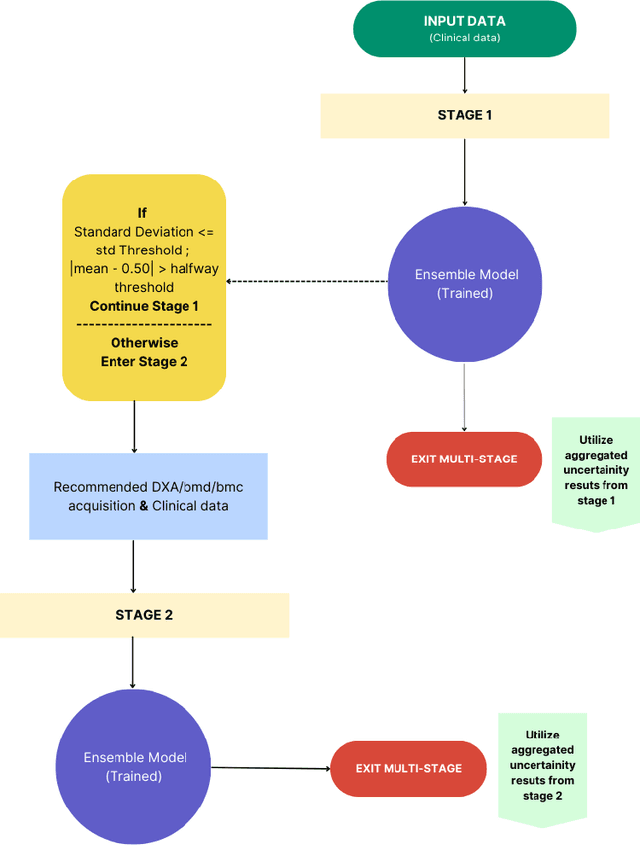
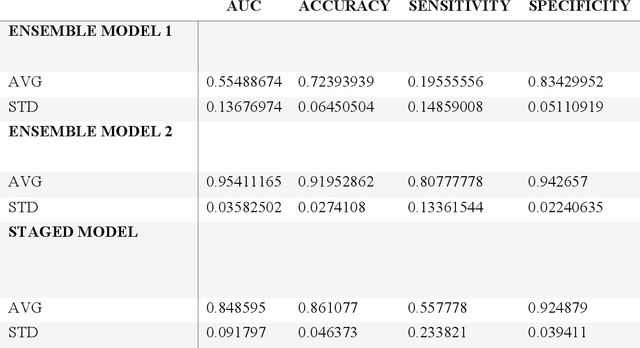
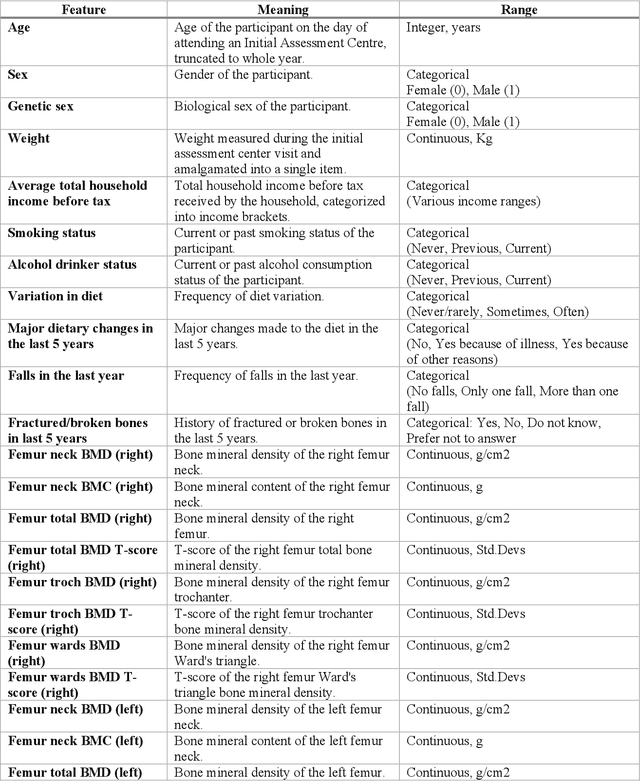
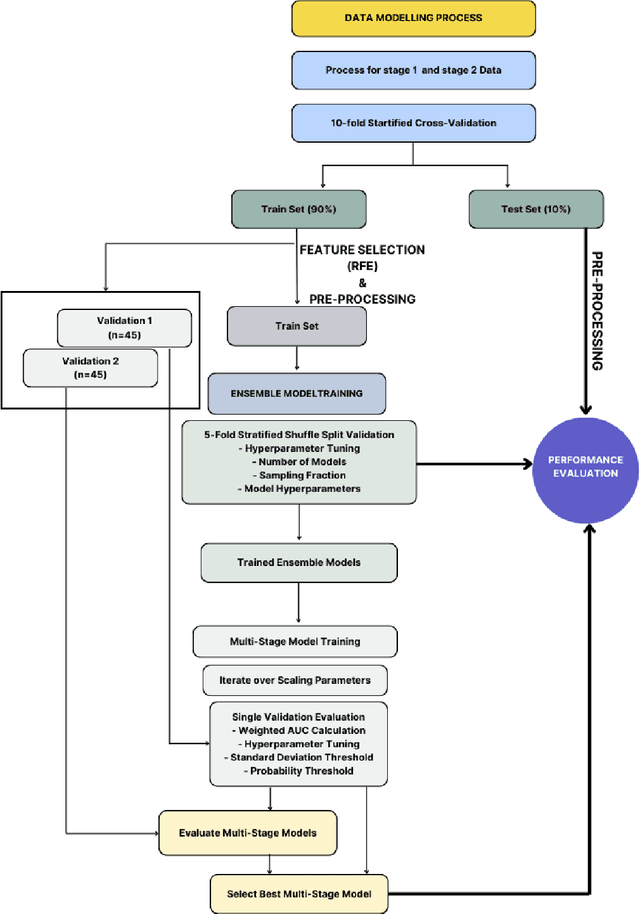
Abstract:Despite advancements in medical care, hip fractures impose a significant burden on individuals and healthcare systems. This paper focuses on the prediction of hip fracture risk in older and middle-aged adults, where falls and compromised bone quality are predominant factors. We propose a novel staged model that combines advanced imaging and clinical data to improve predictive performance. By using CNNs to extract features from hip DXA images, along with clinical variables, shape measurements, and texture features, our method provides a comprehensive framework for assessing fracture risk. A staged machine learning-based model was developed using two ensemble models: Ensemble 1 (clinical variables only) and Ensemble 2 (clinical variables and DXA imaging features). This staged approach used uncertainty quantification from Ensemble 1 to decide if DXA features are necessary for further prediction. Ensemble 2 exhibited the highest performance, achieving an AUC of 0.9541, an accuracy of 0.9195, a sensitivity of 0.8078, and a specificity of 0.9427. The staged model also performed well, with an AUC of 0.8486, an accuracy of 0.8611, a sensitivity of 0.5578, and a specificity of 0.9249, outperforming Ensemble 1, which had an AUC of 0.5549, an accuracy of 0.7239, a sensitivity of 0.1956, and a specificity of 0.8343. Furthermore, the staged model suggested that 54.49% of patients did not require DXA scanning. It effectively balanced accuracy and specificity, offering a robust solution when DXA data acquisition is not always feasible. Statistical tests confirmed significant differences between the models, highlighting the advantages of the advanced modeling strategies. Our staged approach could identify individuals at risk with a high accuracy but reduce the unnecessary DXA scanning. It has great promise to guide interventions to prevent hip fractures with reduced cost and radiation.
A Deep Learning-Based Method for Automatic Segmentation of Proximal Femur from Quantitative Computed Tomography Images
Jul 01, 2020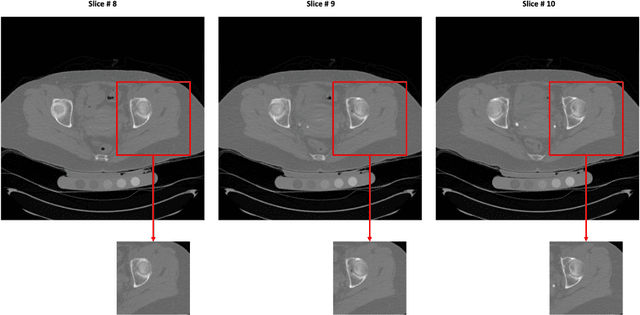
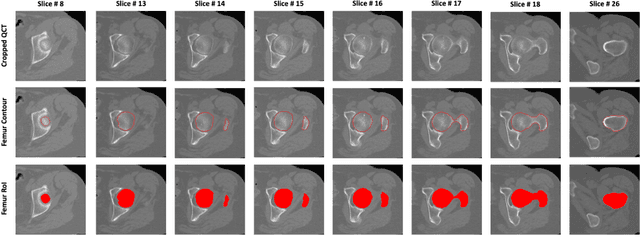
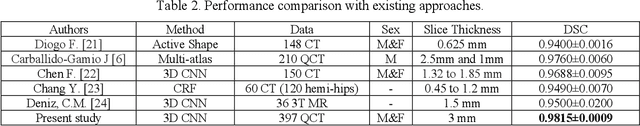
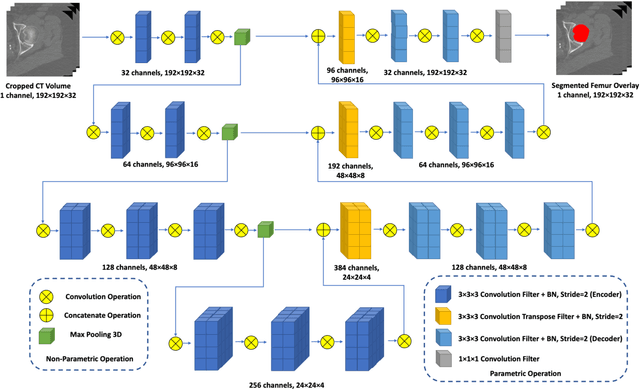
Abstract:Purpose: Proximal femur image analyses based on quantitative computed tomography (QCT) provide a method to quantify the bone density and evaluate osteoporosis and risk of fracture. We aim to develop a deep-learning-based method for automatic proximal femur segmentation. Methods and Materials: We developed a 3D image segmentation method based on V-Net, an end-to-end fully convolutional neural network (CNN), to extract the proximal femur QCT images automatically. The proposed V-net methodology adopts a compound loss function, which includes a Dice loss and a L2 regularizer. We performed experiments to evaluate the effectiveness of the proposed segmentation method. In the experiments, a QCT dataset which included 397 QCT subjects was used. For the QCT image of each subject, the ground truth for the proximal femur was delineated by a well-trained scientist. During the experiments for the entire cohort then for male and female subjects separately, 90% of the subjects were used in 10-fold cross-validation for training and internal validation, and to select the optimal parameters of the proposed models; the rest of the subjects were used to evaluate the performance of models. Results: Visual comparison demonstrated high agreement between the model prediction and ground truth contours of the proximal femur portion of the QCT images. In the entire cohort, the proposed model achieved a Dice score of 0.9815, a sensitivity of 0.9852 and a specificity of 0.9992. In addition, an R2 score of 0.9956 (p<0.001) was obtained when comparing the volumes measured by our model prediction with the ground truth. Conclusion: This method shows a great promise for clinical application to QCT and QCT-based finite element analysis of the proximal femur for evaluating osteoporosis and hip fracture risk.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge