Chaoyang Zhang
A Survey of Scientific Large Language Models: From Data Foundations to Agent Frontiers
Aug 28, 2025



Abstract:Scientific Large Language Models (Sci-LLMs) are transforming how knowledge is represented, integrated, and applied in scientific research, yet their progress is shaped by the complex nature of scientific data. This survey presents a comprehensive, data-centric synthesis that reframes the development of Sci-LLMs as a co-evolution between models and their underlying data substrate. We formulate a unified taxonomy of scientific data and a hierarchical model of scientific knowledge, emphasizing the multimodal, cross-scale, and domain-specific challenges that differentiate scientific corpora from general natural language processing datasets. We systematically review recent Sci-LLMs, from general-purpose foundations to specialized models across diverse scientific disciplines, alongside an extensive analysis of over 270 pre-/post-training datasets, showing why Sci-LLMs pose distinct demands -- heterogeneous, multi-scale, uncertainty-laden corpora that require representations preserving domain invariance and enabling cross-modal reasoning. On evaluation, we examine over 190 benchmark datasets and trace a shift from static exams toward process- and discovery-oriented assessments with advanced evaluation protocols. These data-centric analyses highlight persistent issues in scientific data development and discuss emerging solutions involving semi-automated annotation pipelines and expert validation. Finally, we outline a paradigm shift toward closed-loop systems where autonomous agents based on Sci-LLMs actively experiment, validate, and contribute to a living, evolving knowledge base. Collectively, this work provides a roadmap for building trustworthy, continually evolving artificial intelligence (AI) systems that function as a true partner in accelerating scientific discovery.
Towards Holistic Visual Quality Assessment of AI-Generated Videos: A LLM-Based Multi-Dimensional Evaluation Model
Jun 05, 2025



Abstract:The development of AI-Generated Video (AIGV) technology has been remarkable in recent years, significantly transforming the paradigm of video content production. However, AIGVs still suffer from noticeable visual quality defects, such as noise, blurriness, frame jitter and low dynamic degree, which severely impact the user's viewing experience. Therefore, an effective automatic visual quality assessment is of great importance for AIGV content regulation and generative model improvement. In this work, we decompose the visual quality of AIGVs into three dimensions: technical quality, motion quality, and video semantics. For each dimension, we design corresponding encoder to achieve effective feature representation. Moreover, considering the outstanding performance of large language models (LLMs) in various vision and language tasks, we introduce a LLM as the quality regression module. To better enable the LLM to establish reasoning associations between multi-dimensional features and visual quality, we propose a specially designed multi-modal prompt engineering framework. Additionally, we incorporate LoRA fine-tuning technology during the training phase, allowing the LLM to better adapt to specific tasks. Our proposed method achieved \textbf{second place} in the NTIRE 2025 Quality Assessment of AI-Generated Content Challenge: Track 2 AI Generated video, demonstrating its effectiveness. Codes can be obtained at https://github.com/QiZelu/AIGVEval.
DAPE: Dual-Stage Parameter-Efficient Fine-Tuning for Consistent Video Editing with Diffusion Models
May 11, 2025Abstract:Video generation based on diffusion models presents a challenging multimodal task, with video editing emerging as a pivotal direction in this field. Recent video editing approaches primarily fall into two categories: training-required and training-free methods. While training-based methods incur high computational costs, training-free alternatives often yield suboptimal performance. To address these limitations, we propose DAPE, a high-quality yet cost-effective two-stage parameter-efficient fine-tuning (PEFT) framework for video editing. In the first stage, we design an efficient norm-tuning method to enhance temporal consistency in generated videos. The second stage introduces a vision-friendly adapter to improve visual quality. Additionally, we identify critical shortcomings in existing benchmarks, including limited category diversity, imbalanced object distribution, and inconsistent frame counts. To mitigate these issues, we curate a large dataset benchmark comprising 232 videos with rich annotations and 6 editing prompts, enabling objective and comprehensive evaluation of advanced methods. Extensive experiments on existing datasets (BalanceCC, LOVEU-TGVE, RAVE) and our proposed benchmark demonstrate that DAPE significantly improves temporal coherence and text-video alignment while outperforming previous state-of-the-art approaches.
Learning-Based Multi-Criteria Decision Model for Site Selection Problems
Apr 05, 2025



Abstract:Strategically locating sawmills is critical for the efficiency, profitability, and sustainability of timber supply chains, yet it involves a series of complex decision-making affected by various factors, such as proximity to resources and markets, proximity to roads and rail lines, distance from the urban area, slope, labor market, and existing sawmill data. Although conventional Multi-Criteria Decision-Making (MCDM) approaches utilize these factors while locating facilities, they are susceptible to bias since they rely heavily on expert opinions to determine the relative factor weights. Machine learning (ML) models provide an objective, data-driven alternative for site selection that derives these weights directly from the patterns in large datasets without requiring subjective weighting. Additionally, ML models autonomously identify critical features, eliminating the need for subjective feature selection. In this study, we propose integrated ML and MCDM methods and showcase the utility of this integrated model to improve sawmill location decisions via a case study in Mississippi. This integrated model is flexible and applicable to site selection problems across various industries.
S2MS: Self-Supervised Learning Driven Multi-Spectral CT Image Enhancement
Jan 26, 2022



Abstract:Photon counting spectral CT (PCCT) can produce reconstructed attenuation maps in different energy channels, reflecting energy properties of the scanned object. Due to the limited photon numbers and the non-ideal detector response of each energy channel, the reconstructed images usually contain much noise. With the development of Deep Learning (DL) technique, different kinds of DL-based models have been proposed for noise reduction. However, most of the models require clean data set as the training labels, which are not always available in medical imaging field. Inspiring by the similarities of each channel's reconstructed image, we proposed a self-supervised learning based PCCT image enhancement framework via multi-spectral channels (S2MS). In S2MS framework, both the input and output labels are noisy images. Specifically, one single channel image was used as output while images of other single channels and channel-sum image were used as input to train the network, which can fully use the spectral data information without extra cost. The simulation results based on the AAPM Low-dose CT Challenge database showed that the proposed S2MS model can suppress the noise and preserve details more effectively in comparison with the traditional DL models, which has potential to improve the image quality of PCCT in clinical applications.
A new approach to extracting coronary arteries and detecting stenosis in invasive coronary angiograms
Jan 25, 2021



Abstract:In stable coronary artery disease (CAD), reduction in mortality and/or myocardial infarction with revascularization over medical therapy has not been reliably achieved. Coronary arteries are usually extracted to perform stenosis detection. We aim to develop an automatic algorithm by deep learning to extract coronary arteries from ICAs.In this study, a multi-input and multi-scale (MIMS) U-Net with a two-stage recurrent training strategy was proposed for the automatic vessel segmentation. Incorporating features such as the Inception residual module with depth-wise separable convolutional layers, the proposed model generated a refined prediction map with the following two training stages: (i) Stage I coarsely segmented the major coronary arteries from pre-processed single-channel ICAs and generated the probability map of vessels; (ii) during the Stage II, a three-channel image consisting of the original preprocessed image, a generated probability map, and an edge-enhanced image generated from the preprocessed image was fed to the proposed MIMS U-Net to produce the final segmentation probability map. During the training stage, the probability maps were iteratively and recurrently updated by feeding into the neural network. After segmentation, an arterial stenosis detection algorithm was developed to extract vascular centerlines and calculate arterial diameters to evaluate stenotic level. Experimental results demonstrated that the proposed method achieved an average Dice score of 0.8329, an average sensitivity of 0.8281, and an average specificity of 0.9979 in our dataset with 294 ICAs obtained from 73 patient. Moreover, our stenosis detection algorithm achieved a true positive rate of 0.6668 and a positive predictive value of 0.7043.
A Deep Learning-Based Method for Automatic Segmentation of Proximal Femur from Quantitative Computed Tomography Images
Jul 01, 2020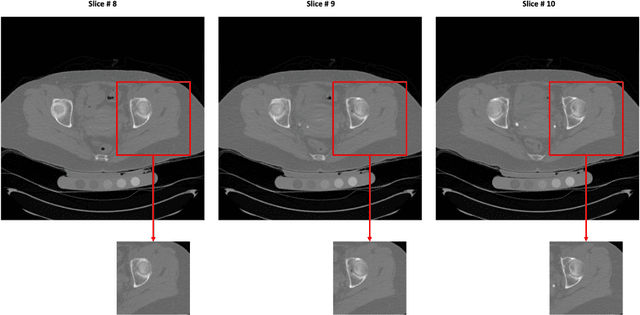
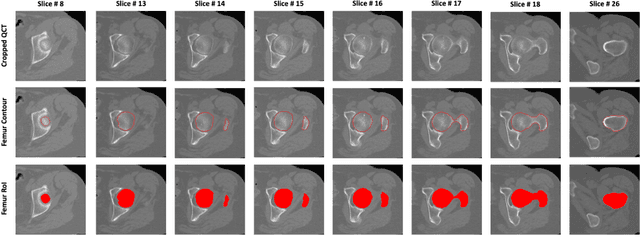
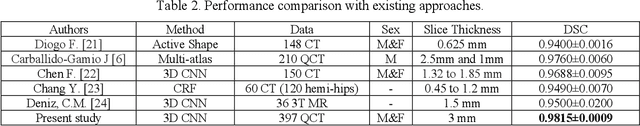
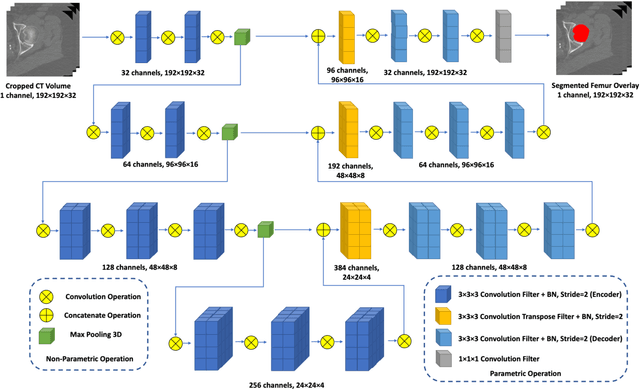
Abstract:Purpose: Proximal femur image analyses based on quantitative computed tomography (QCT) provide a method to quantify the bone density and evaluate osteoporosis and risk of fracture. We aim to develop a deep-learning-based method for automatic proximal femur segmentation. Methods and Materials: We developed a 3D image segmentation method based on V-Net, an end-to-end fully convolutional neural network (CNN), to extract the proximal femur QCT images automatically. The proposed V-net methodology adopts a compound loss function, which includes a Dice loss and a L2 regularizer. We performed experiments to evaluate the effectiveness of the proposed segmentation method. In the experiments, a QCT dataset which included 397 QCT subjects was used. For the QCT image of each subject, the ground truth for the proximal femur was delineated by a well-trained scientist. During the experiments for the entire cohort then for male and female subjects separately, 90% of the subjects were used in 10-fold cross-validation for training and internal validation, and to select the optimal parameters of the proposed models; the rest of the subjects were used to evaluate the performance of models. Results: Visual comparison demonstrated high agreement between the model prediction and ground truth contours of the proximal femur portion of the QCT images. In the entire cohort, the proposed model achieved a Dice score of 0.9815, a sensitivity of 0.9852 and a specificity of 0.9992. In addition, an R2 score of 0.9956 (p<0.001) was obtained when comparing the volumes measured by our model prediction with the ground truth. Conclusion: This method shows a great promise for clinical application to QCT and QCT-based finite element analysis of the proximal femur for evaluating osteoporosis and hip fracture risk.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge