Haipeng Tang
Automatic Identification of the End-Diastolic and End-Systolic Cardiac Frames from Invasive Coronary Angiography Videos
Oct 06, 2021

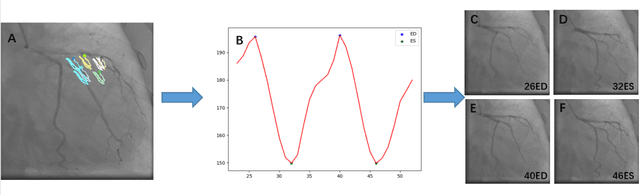
Abstract:Automatic identification of proper image frames at the end-diastolic (ED) and end-systolic (ES) frames during the review of invasive coronary angiograms (ICA) is important to assess blood flow during a cardiac cycle, reconstruct the 3D arterial anatomy from bi-planar views, and generate the complementary fusion map with myocardial images. The current identification method primarily relies on visual interpretation, making it not only time-consuming but also less reproducible. In this paper, we propose a new method to automatically identify angiographic image frames associated with the ED and ES cardiac phases by using the trajectories of key vessel points (i.e. landmarks). More specifically, a detection algorithm is first used to detect the key points of coronary arteries, and then an optical flow method is employed to track the trajectories of the selected key points. The ED and ES frames are identified based on all these trajectories. Our method was tested with 62 ICA videos from two separate medical centers (22 and 9 patients in sites 1 and 2, respectively). Comparing consensus interpretations by two human expert readers, excellent agreement was achieved by the proposed algorithm: the agreement rates within a one-frame range were 92.99% and 92.73% for the automatic identification of the ED and ES image frames, respectively. In conclusion, the proposed automated method showed great potential for being an integral part of automated ICA image analysis.
A new approach to extracting coronary arteries and detecting stenosis in invasive coronary angiograms
Jan 25, 2021



Abstract:In stable coronary artery disease (CAD), reduction in mortality and/or myocardial infarction with revascularization over medical therapy has not been reliably achieved. Coronary arteries are usually extracted to perform stenosis detection. We aim to develop an automatic algorithm by deep learning to extract coronary arteries from ICAs.In this study, a multi-input and multi-scale (MIMS) U-Net with a two-stage recurrent training strategy was proposed for the automatic vessel segmentation. Incorporating features such as the Inception residual module with depth-wise separable convolutional layers, the proposed model generated a refined prediction map with the following two training stages: (i) Stage I coarsely segmented the major coronary arteries from pre-processed single-channel ICAs and generated the probability map of vessels; (ii) during the Stage II, a three-channel image consisting of the original preprocessed image, a generated probability map, and an edge-enhanced image generated from the preprocessed image was fed to the proposed MIMS U-Net to produce the final segmentation probability map. During the training stage, the probability maps were iteratively and recurrently updated by feeding into the neural network. After segmentation, an arterial stenosis detection algorithm was developed to extract vascular centerlines and calculate arterial diameters to evaluate stenotic level. Experimental results demonstrated that the proposed method achieved an average Dice score of 0.8329, an average sensitivity of 0.8281, and an average specificity of 0.9979 in our dataset with 294 ICAs obtained from 73 patient. Moreover, our stenosis detection algorithm achieved a true positive rate of 0.6668 and a positive predictive value of 0.7043.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge