Jin Jing
EGS-SLAM: RGB-D Gaussian Splatting SLAM with Events
Aug 09, 2025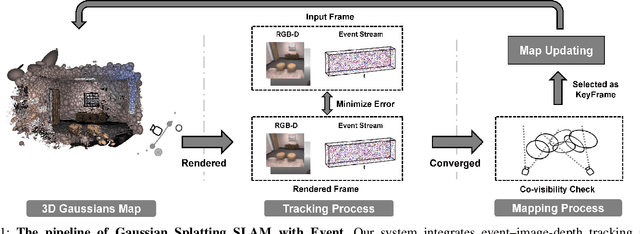
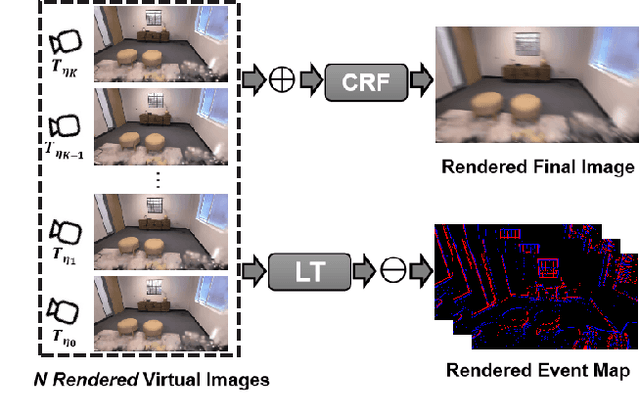


Abstract:Gaussian Splatting SLAM (GS-SLAM) offers a notable improvement over traditional SLAM methods, enabling photorealistic 3D reconstruction that conventional approaches often struggle to achieve. However, existing GS-SLAM systems perform poorly under persistent and severe motion blur commonly encountered in real-world scenarios, leading to significantly degraded tracking accuracy and compromised 3D reconstruction quality. To address this limitation, we propose EGS-SLAM, a novel GS-SLAM framework that fuses event data with RGB-D inputs to simultaneously reduce motion blur in images and compensate for the sparse and discrete nature of event streams, enabling robust tracking and high-fidelity 3D Gaussian Splatting reconstruction. Specifically, our system explicitly models the camera's continuous trajectory during exposure, supporting event- and blur-aware tracking and mapping on a unified 3D Gaussian Splatting scene. Furthermore, we introduce a learnable camera response function to align the dynamic ranges of events and images, along with a no-event loss to suppress ringing artifacts during reconstruction. We validate our approach on a new dataset comprising synthetic and real-world sequences with significant motion blur. Extensive experimental results demonstrate that EGS-SLAM consistently outperforms existing GS-SLAM systems in both trajectory accuracy and photorealistic 3D Gaussian Splatting reconstruction. The source code will be available at https://github.com/Chensiyu00/EGS-SLAM.
Mapping the Ictal-Interictal-Injury Continuum Using Interpretable Machine Learning
Nov 14, 2022


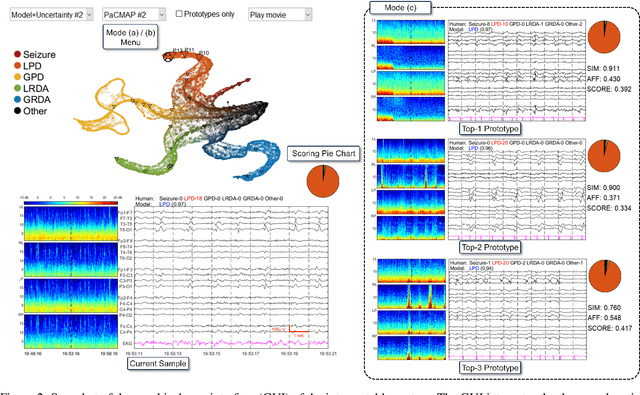
Abstract:IMPORTANCE: An interpretable machine learning model can provide faithful explanations of each prediction and yet maintain higher performance than its black box counterpart. OBJECTIVE: To design an interpretable machine learning model which accurately predicts EEG protopatterns while providing an explanation of its predictions with assistance of a specialized GUI. To map the cEEG latent features to a 2D space in order to visualize the ictal-interictal-injury continuum and gain insight into its high-dimensional structure. DESIGN, SETTING, AND PARTICIPANTS: 50,697 50-second cEEG samples from 2,711 ICU patients collected between July 2006 and March 2020 at Massachusetts General Hospital. Samples were labeled as one of 6 EEG activities by domain experts, with 124 different experts providing annotations. MAIN OUTCOMES AND MEASURES: Our neural network is interpretable because it uses case-based reasoning: it compares a new EEG reading to a set of learned prototypical EEG samples from the training dataset. Interpretability was measured with task-specific neighborhood agreement statistics. Discriminatory performance was evaluated with AUROC and AUPRC. RESULTS: The model achieves AUROCs of 0.87, 0.93, 0.96, 0.92, 0.93, 0.80 for classes Seizure, LPD, GPD, LRDA, GRDA, Other respectively. This performance is statistically significantly higher than that of the corresponding uninterpretable (black box) model with p<0.0001. Videos of the ictal-interictal-injury continuum are provided. CONCLUSION AND RELEVANCE: Our interpretable model and GUI can act as a reference for practitioners who work with cEEG patterns. We can now better understand the relationships between different types of cEEG patterns. In the future, this system may allow for targeted intervention and training in clinical settings. It could also be used for re-confirming or providing additional information for diagnostics.
Why Interpretable Causal Inference is Important for High-Stakes Decision Making for Critically Ill Patients and How To Do It
Mar 09, 2022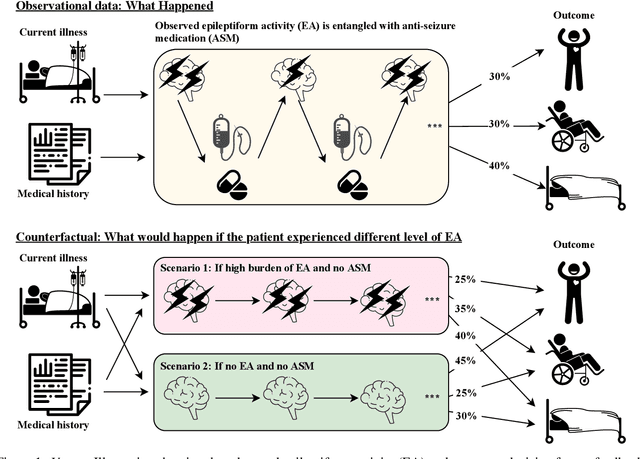
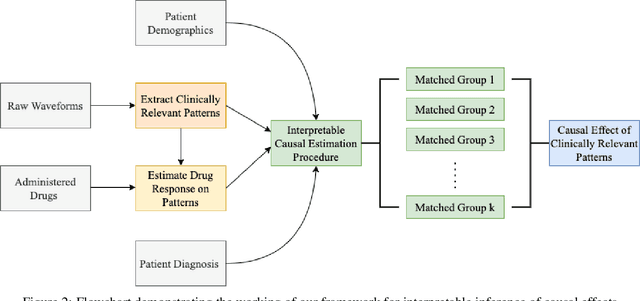
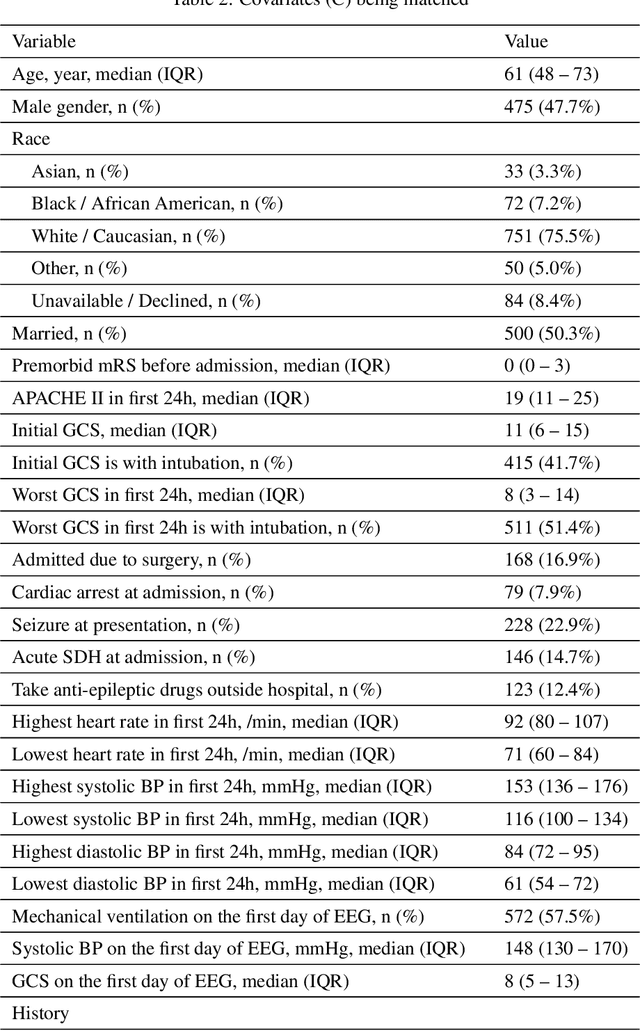
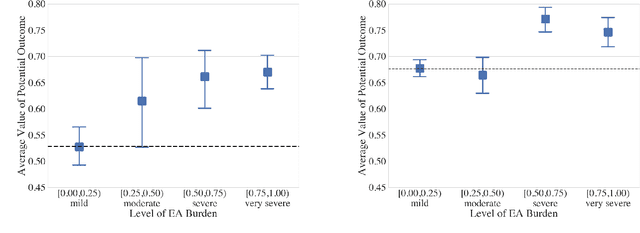
Abstract:Many fundamental problems affecting the care of critically ill patients lead to similar analytical challenges: physicians cannot easily estimate the effects of at-risk medical conditions or treatments because the causal effects of medical conditions and drugs are entangled. They also cannot easily perform studies: there are not enough high-quality data for high-dimensional observational causal inference, and RCTs often cannot ethically be conducted. However, mechanistic knowledge is available, including how drugs are absorbed into the body, and the combination of this knowledge with the limited data could potentially suffice -- if we knew how to combine them. In this work, we present a framework for interpretable estimation of causal effects for critically ill patients under exactly these complex conditions: interactions between drugs and observations over time, patient data sets that are not large, and mechanistic knowledge that can substitute for lack of data. We apply this framework to an extremely important problem affecting critically ill patients, namely the effect of seizures and other potentially harmful electrical events in the brain (called epileptiform activity -- EA) on outcomes. Given the high stakes involved and the high noise in the data, interpretability is critical for troubleshooting such complex problems. Interpretability of our matched groups allowed neurologists to perform chart reviews to verify the quality of our causal analysis. For instance, our work indicates that a patient who experiences a high level of seizure-like activity (75% high EA burden) and is untreated for a six-hour window, has, on average, a 16.7% increased chance of adverse outcomes such as severe brain damage, lifetime disability, or death. We find that patients with mild but long-lasting EA (average EA burden >= 50%) have their risk of an adverse outcome increased by 11.2%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge