Kentaro Hoffman
Unique Rashomon Sets for Robust Active Learning
Mar 09, 2025



Abstract:Collecting labeled data for machine learning models is often expensive and time-consuming. Active learning addresses this challenge by selectively labeling the most informative observations, but when initial labeled data is limited, it becomes difficult to distinguish genuinely informative points from those appearing uncertain primarily due to noise. Ensemble methods like random forests are a powerful approach to quantifying this uncertainty but do so by aggregating all models indiscriminately. This includes poor performing models and redundant models, a problem that worsens in the presence of noisy data. We introduce UNique Rashomon Ensembled Active Learning (UNREAL), which selectively ensembles only distinct models from the Rashomon set, which is the set of nearly optimal models. Restricting ensemble membership to high-performing models with different explanations helps distinguish genuine uncertainty from noise-induced variation. We show that UNREAL achieves faster theoretical convergence rates than traditional active learning approaches and demonstrates empirical improvements of up to 20% in predictive accuracy across five benchmark datasets, while simultaneously enhancing model interpretability.
From Narratives to Numbers: Valid Inference Using Language Model Predictions from Verbal Autopsy Narratives
Apr 03, 2024



Abstract:In settings where most deaths occur outside the healthcare system, verbal autopsies (VAs) are a common tool to monitor trends in causes of death (COD). VAs are interviews with a surviving caregiver or relative that are used to predict the decedent's COD. Turning VAs into actionable insights for researchers and policymakers requires two steps (i) predicting likely COD using the VA interview and (ii) performing inference with predicted CODs (e.g. modeling the breakdown of causes by demographic factors using a sample of deaths). In this paper, we develop a method for valid inference using outcomes (in our case COD) predicted from free-form text using state-of-the-art NLP techniques. This method, which we call multiPPI++, extends recent work in "prediction-powered inference" to multinomial classification. We leverage a suite of NLP techniques for COD prediction and, through empirical analysis of VA data, demonstrate the effectiveness of our approach in handling transportability issues. multiPPI++ recovers ground truth estimates, regardless of which NLP model produced predictions and regardless of whether they were produced by a more accurate predictor like GPT-4-32k or a less accurate predictor like KNN. Our findings demonstrate the practical importance of inference correction for public health decision-making and suggests that if inference tasks are the end goal, having a small amount of contextually relevant, high quality labeled data is essential regardless of the NLP algorithm.
Do We Really Even Need Data?
Feb 02, 2024Abstract:As artificial intelligence and machine learning tools become more accessible, and scientists face new obstacles to data collection (e.g. rising costs, declining survey response rates), researchers increasingly use predictions from pre-trained algorithms as outcome variables. Though appealing for financial and logistical reasons, using standard tools for inference can misrepresent the association between independent variables and the outcome of interest when the true, unobserved outcome is replaced by a predicted value. In this paper, we characterize the statistical challenges inherent to this so-called ``inference with predicted data'' problem and elucidate three potential sources of error: (i) the relationship between predicted outcomes and their true, unobserved counterparts, (ii) robustness of the machine learning model to resampling or uncertainty about the training data, and (iii) appropriately propagating not just bias but also uncertainty from predictions into the ultimate inference procedure.
Why Interpretable Causal Inference is Important for High-Stakes Decision Making for Critically Ill Patients and How To Do It
Mar 09, 2022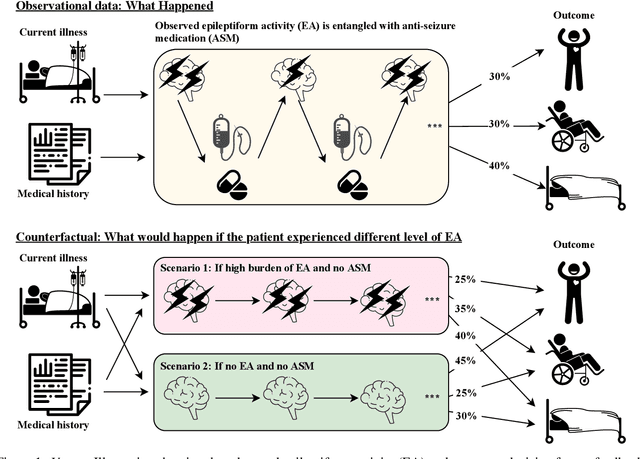
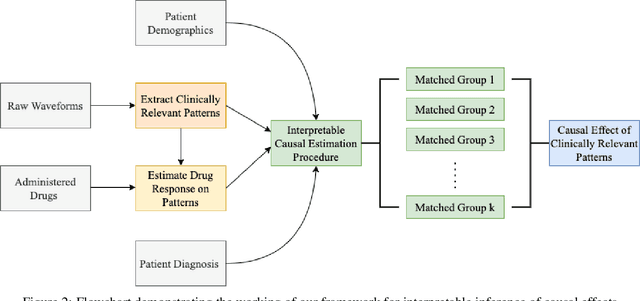
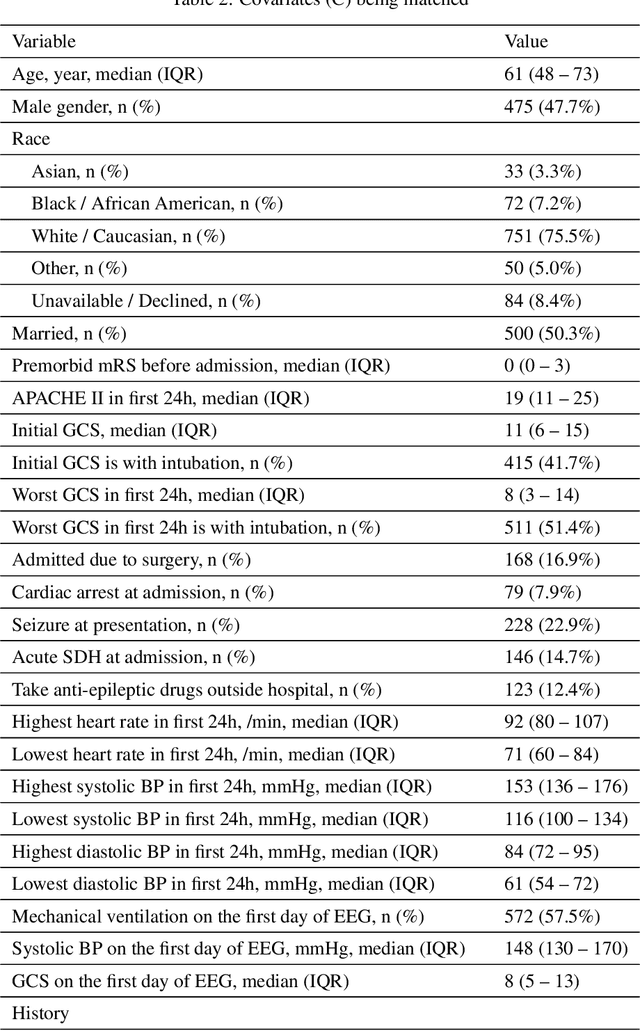
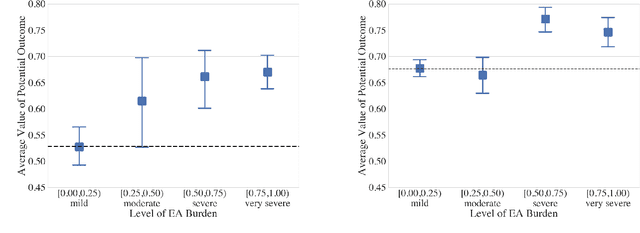
Abstract:Many fundamental problems affecting the care of critically ill patients lead to similar analytical challenges: physicians cannot easily estimate the effects of at-risk medical conditions or treatments because the causal effects of medical conditions and drugs are entangled. They also cannot easily perform studies: there are not enough high-quality data for high-dimensional observational causal inference, and RCTs often cannot ethically be conducted. However, mechanistic knowledge is available, including how drugs are absorbed into the body, and the combination of this knowledge with the limited data could potentially suffice -- if we knew how to combine them. In this work, we present a framework for interpretable estimation of causal effects for critically ill patients under exactly these complex conditions: interactions between drugs and observations over time, patient data sets that are not large, and mechanistic knowledge that can substitute for lack of data. We apply this framework to an extremely important problem affecting critically ill patients, namely the effect of seizures and other potentially harmful electrical events in the brain (called epileptiform activity -- EA) on outcomes. Given the high stakes involved and the high noise in the data, interpretability is critical for troubleshooting such complex problems. Interpretability of our matched groups allowed neurologists to perform chart reviews to verify the quality of our causal analysis. For instance, our work indicates that a patient who experiences a high level of seizure-like activity (75% high EA burden) and is untreated for a six-hour window, has, on average, a 16.7% increased chance of adverse outcomes such as severe brain damage, lifetime disability, or death. We find that patients with mild but long-lasting EA (average EA burden >= 50%) have their risk of an adverse outcome increased by 11.2%.
Local Change Point Detection and Signal Cleaning using EEMD with applications to Acoustic Shockwaves and Cardiac Signals
Mar 01, 2021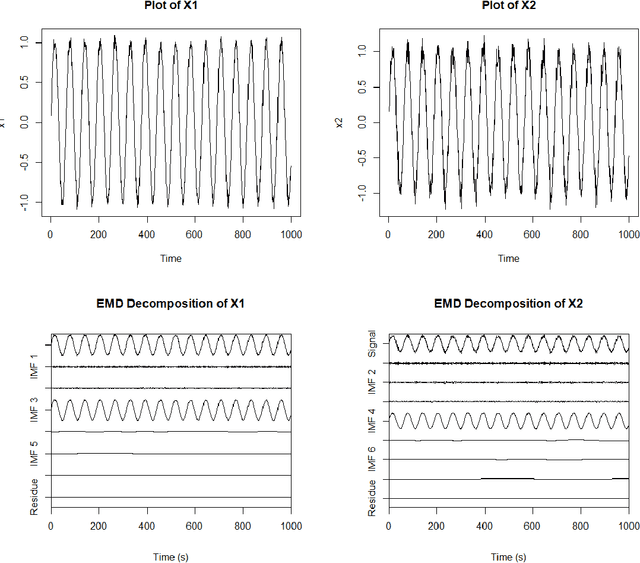



Abstract:With the ability to create time varying basis functions, the Ensemble Empirical Mode Decomposition (EEMD) has quickly become the preferred way to decompose nonlinear and nonstationary signals. However, we find current EEMD signal cleaning techniques lacking, unable to deal with the nonlinearities that are common for the complex signals that the EEMD is used for. By combining change point detection and a new sparse basis function optimization problem, we are able to show that it is possible to create unique filters for each change point which emphasize the basis functions that are observing a change. This not only allows one to understand which frequency bands are observing a change, but cleaning the signal to emphasize changes can lead to improved signal classification accuracy. We show that this technique has implications for a variety of applications including acoustics and medicine. The technique is implemented in R via the \textbf{LCDSC} package.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge