Jeffrey B. Ware
BraTS-PEDs: Results of the Multi-Consortium International Pediatric Brain Tumor Segmentation Challenge 2023
Jul 11, 2024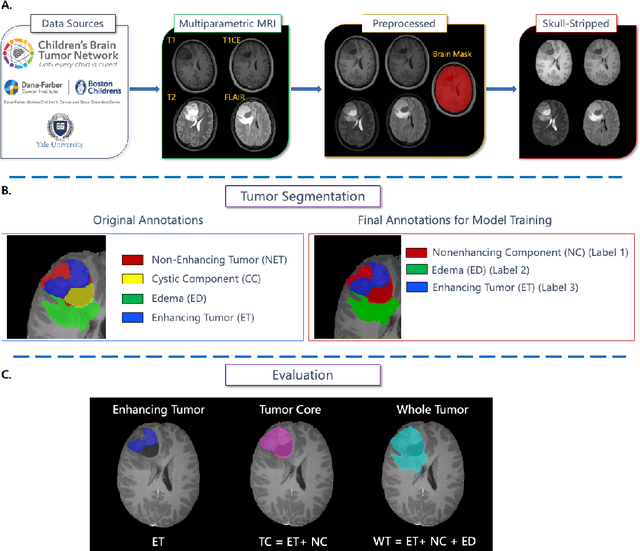

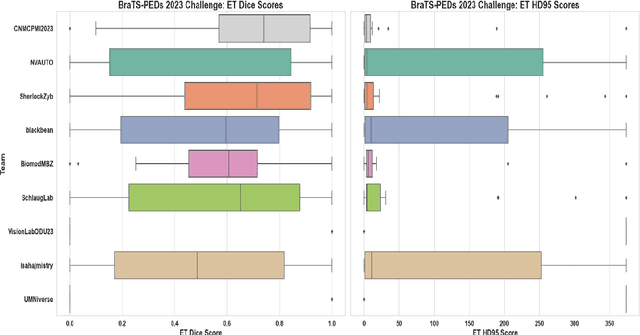
Abstract:Pediatric central nervous system tumors are the leading cause of cancer-related deaths in children. The five-year survival rate for high-grade glioma in children is less than 20%. The development of new treatments is dependent upon multi-institutional collaborative clinical trials requiring reproducible and accurate centralized response assessment. We present the results of the BraTS-PEDs 2023 challenge, the first Brain Tumor Segmentation (BraTS) challenge focused on pediatric brain tumors. This challenge utilized data acquired from multiple international consortia dedicated to pediatric neuro-oncology and clinical trials. BraTS-PEDs 2023 aimed to evaluate volumetric segmentation algorithms for pediatric brain gliomas from magnetic resonance imaging using standardized quantitative performance evaluation metrics employed across the BraTS 2023 challenges. The top-performing AI approaches for pediatric tumor analysis included ensembles of nnU-Net and Swin UNETR, Auto3DSeg, or nnU-Net with a self-supervised framework. The BraTSPEDs 2023 challenge fostered collaboration between clinicians (neuro-oncologists, neuroradiologists) and AI/imaging scientists, promoting faster data sharing and the development of automated volumetric analysis techniques. These advancements could significantly benefit clinical trials and improve the care of children with brain tumors.
Training and Comparison of nnU-Net and DeepMedic Methods for Autosegmentation of Pediatric Brain Tumors
Jan 30, 2024



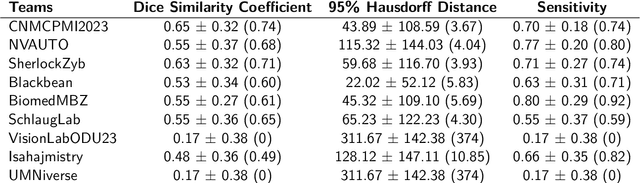
Abstract:Brain tumors are the most common solid tumors and the leading cause of cancer-related death among children. Tumor segmentation is essential in surgical and treatment planning, and response assessment and monitoring. However, manual segmentation is time-consuming and has high inter-operator variability, underscoring the need for more efficient methods. We compared two deep learning-based 3D segmentation models, DeepMedic and nnU-Net, after training with pediatric-specific multi-institutional brain tumor data using based on multi-parametric MRI scans.Multi-parametric preoperative MRI scans of 339 pediatric patients (n=293 internal and n=46 external cohorts) with a variety of tumor subtypes, were preprocessed and manually segmented into four tumor subregions, i.e., enhancing tumor (ET), non-enhancing tumor (NET), cystic components (CC), and peritumoral edema (ED). After training, performance of the two models on internal and external test sets was evaluated using Dice scores, sensitivity, and Hausdorff distance with reference to ground truth manual segmentations. Dice score for nnU-Net internal test sets was (mean +/- SD (median)) 0.9+/-0.07 (0.94) for WT, 0.77+/-0.29 for ET, 0.66+/-0.32 for NET, 0.71+/-0.33 for CC, and 0.71+/-0.40 for ED, respectively. For DeepMedic the Dice scores were 0.82+/-0.16 for WT, 0.66+/-0.32 for ET, 0.48+/-0.27, for NET, 0.48+/-0.36 for CC, and 0.19+/-0.33 for ED, respectively. Dice scores were significantly higher for nnU-Net (p<=0.01). External validation of the trained nnU-Net model on the multi-institutional BraTS-PEDs 2023 dataset revealed high generalization capability in segmentation of whole tumor and tumor core with Dice scores of 0.87+/-0.13 (0.91) and 0.83+/-0.18 (0.89), respectively. Pediatric-specific data trained nnU-Net model is superior to DeepMedic for whole tumor and subregion segmentation of pediatric brain tumors.
A multi-institutional pediatric dataset of clinical radiology MRIs by the Children's Brain Tumor Network
Oct 02, 2023

Abstract:Pediatric brain and spinal cancers remain the leading cause of cancer-related death in children. Advancements in clinical decision-support in pediatric neuro-oncology utilizing the wealth of radiology imaging data collected through standard care, however, has significantly lagged other domains. Such data is ripe for use with predictive analytics such as artificial intelligence (AI) methods, which require large datasets. To address this unmet need, we provide a multi-institutional, large-scale pediatric dataset of 23,101 multi-parametric MRI exams acquired through routine care for 1,526 brain tumor patients, as part of the Children's Brain Tumor Network. This includes longitudinal MRIs across various cancer diagnoses, with associated patient-level clinical information, digital pathology slides, as well as tissue genotype and omics data. To facilitate downstream analysis, treatment-na\"ive images for 370 subjects were processed and released through the NCI Childhood Cancer Data Initiative via the Cancer Data Service. Through ongoing efforts to continuously build these imaging repositories, our aim is to accelerate discovery and translational AI models with real-world data, to ultimately empower precision medicine for children.
Deep Learning Based Detection of Enlarged Perivascular Spaces on Brain MRI
Sep 27, 2022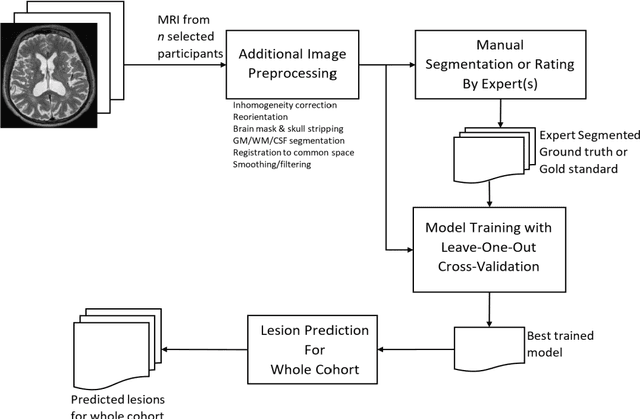
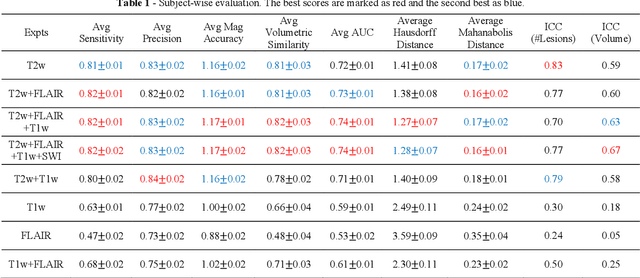
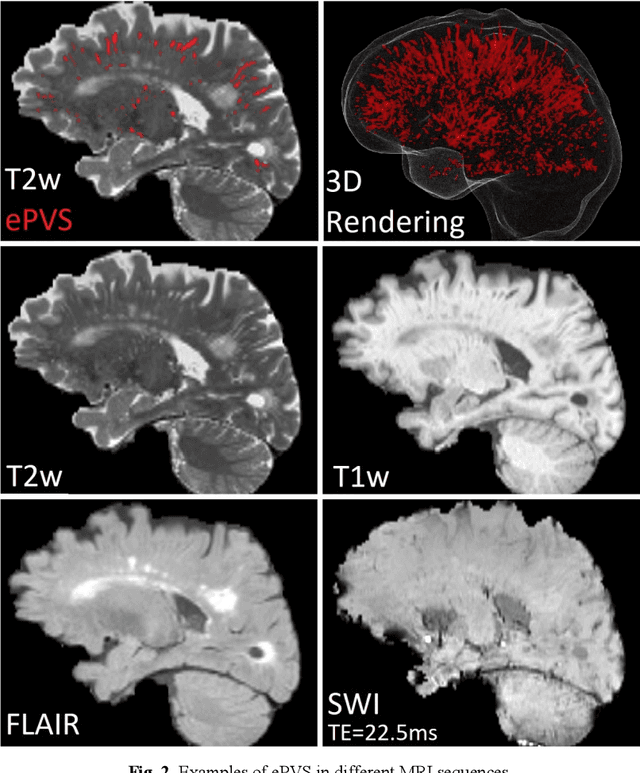
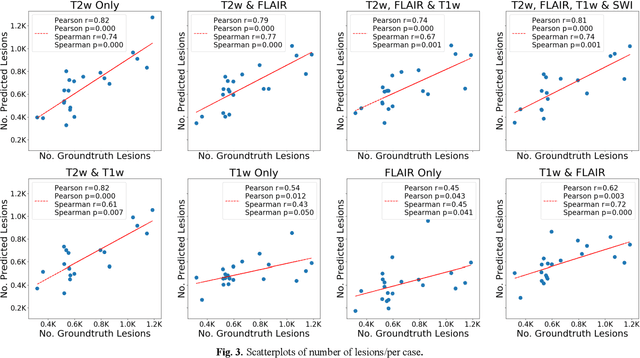
Abstract:Deep learning has been demonstrated effective in many neuroimaging applications. However, in many scenarios the number of imaging sequences capturing information related to small vessel disease lesions is insufficient to support data-driven techniques. Additionally, cohort-based studies may not always have the optimal or essential imaging sequences for accurate lesion detection. Therefore, it is necessary to determine which of these imaging sequences are essential for accurate detection. In this study we aimed to find the optimal combination of magnetic resonance imaging (MRI) sequences for deep learning-based detection of enlarged perivascular spaces (ePVS). To this end, we implemented an effective light-weight U-Net adapted for ePVS detection and comprehensively investigated different combinations of information from susceptibility weighted imaging (SWI), fluid-attenuated inversion recovery (FLAIR), T1-weighted (T1w) and T2-weighted (T2w) MRI sequences. We conclude that T2w MRI is the most important for accurate ePVS detection, and the incorporation of SWI, FLAIR and T1w MRI in the deep neural network could make insignificant improvements in accuracy.
DEEPMIR: A DEEP convolutional neural network for differential detection of cerebral Microbleeds and IRon deposits in MRI
Sep 30, 2020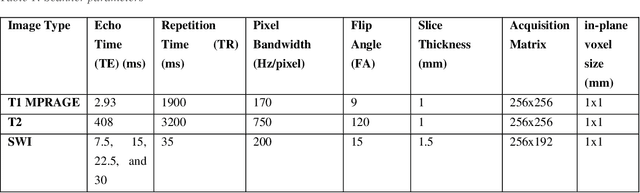
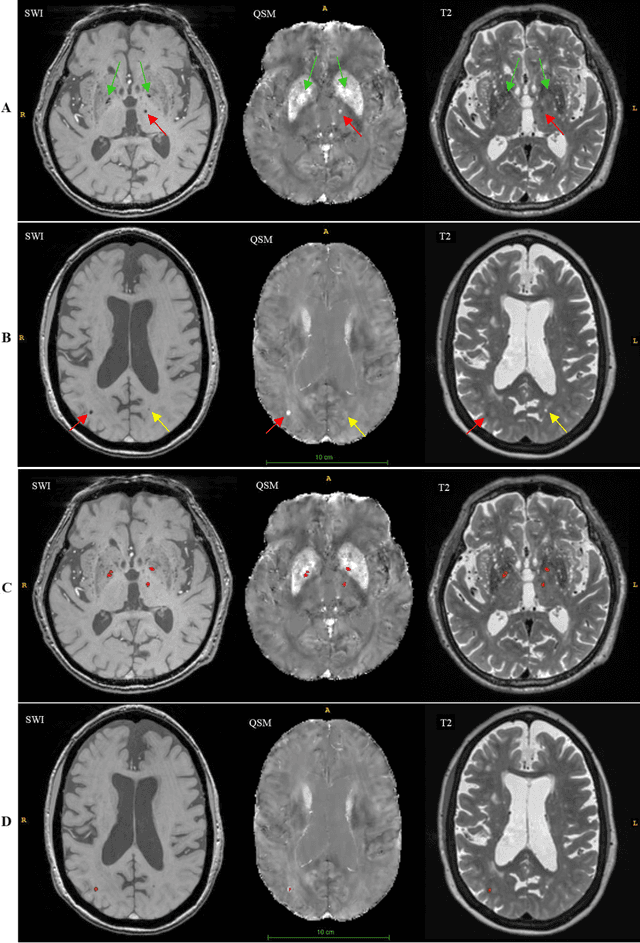
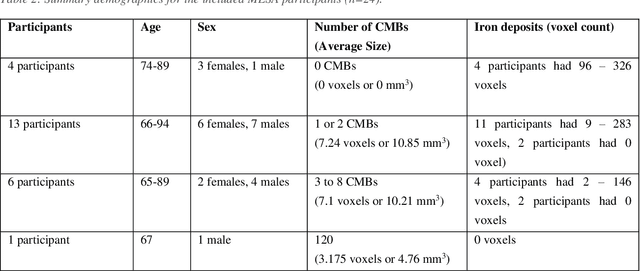

Abstract:Background: Cerebral microbleeds (CMBs) and non-hemorrhage iron deposits in the basal ganglia have been associated with brain aging, vascular disease and neurodegenerative disorders. Recent advances using quantitative susceptibility mapping (QSM) make it possible to differentiate iron content from mineralization in-vivo using magnetic resonance imaging (MRI). However, automated detection of such lesions is still challenging, making quantification in large cohort bases studies rather limited. Purpose: Development of a fully automated method using deep learning for detecting CMBs and basal ganglia iron deposits using multimodal MRI. Materials and Methods: We included a convenience sample of 24 participants from the MESA cohort and used T2-weighted images, susceptibility weighted imaging (SWI), and QSM to segment the lesions. We developed a protocol for simultaneous manual annotation of CMBs and non-hemorrhage iron deposits in the basal ganglia, which resulted in defining the gold standard. This gold standard was then used to train a deep convolution neural network (CNN) model. Specifically, we adapted the U-Net model with a higher number of resolution layers to be able to detect small lesions such as CMBs from standard resolution MRI which are used in cohort-based studies. The detection performance was then evaluated using the cross-validation principle in order to ensure generalization of the results. Results: With multi-class CNN models, we achieved an average sensitivity and precision of about 0.8 and 0.6, respectively for detecting CMBs. The same framework detected non-hemorrhage iron deposits reaching an average sensitivity and precision of about 0.8. Conclusions: Our results showed that deep learning could automate the detection of small vessel disease lesions and including multimodal MR data such as QSM can improve the detection of CMB and non-hemorrhage iron deposits.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge