Fabian Eitel
Benchmark data to study the influence of pre-training on explanation performance in MR image classification
Jun 21, 2023



Abstract:Convolutional Neural Networks (CNNs) are frequently and successfully used in medical prediction tasks. They are often used in combination with transfer learning, leading to improved performance when training data for the task are scarce. The resulting models are highly complex and typically do not provide any insight into their predictive mechanisms, motivating the field of 'explainable' artificial intelligence (XAI). However, previous studies have rarely quantitatively evaluated the 'explanation performance' of XAI methods against ground-truth data, and transfer learning and its influence on objective measures of explanation performance has not been investigated. Here, we propose a benchmark dataset that allows for quantifying explanation performance in a realistic magnetic resonance imaging (MRI) classification task. We employ this benchmark to understand the influence of transfer learning on the quality of explanations. Experimental results show that popular XAI methods applied to the same underlying model differ vastly in performance, even when considering only correctly classified examples. We further observe that explanation performance strongly depends on the task used for pre-training and the number of CNN layers pre-trained. These results hold after correcting for a substantial correlation between explanation and classification performance.
Promises and pitfalls of deep neural networks in neuroimaging-based psychiatric research
Jan 20, 2023



Abstract:By promising more accurate diagnostics and individual treatment recommendations, deep neural networks and in particular convolutional neural networks have advanced to a powerful tool in medical imaging. Here, we first give an introduction into methodological key concepts and resulting methodological promises including representation and transfer learning, as well as modelling domain-specific priors. After reviewing recent applications within neuroimaging-based psychiatric research, such as the diagnosis of psychiatric diseases, delineation of disease subtypes, normative modeling, and the development of neuroimaging biomarkers, we discuss current challenges. This includes for example the difficulty of training models on small, heterogeneous and biased data sets, the lack of validity of clinical labels, algorithmic bias, and the influence of confounding variables.
Feature visualization for convolutional neural network models trained on neuroimaging data
Mar 24, 2022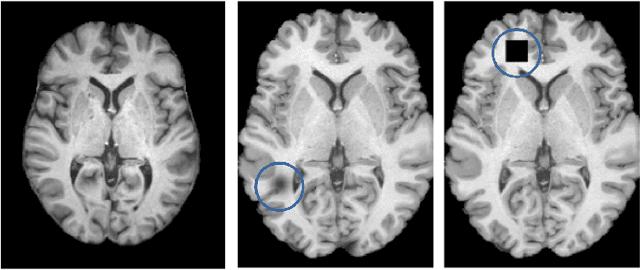
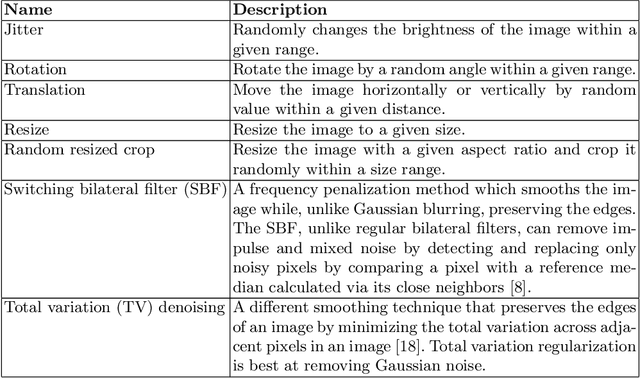
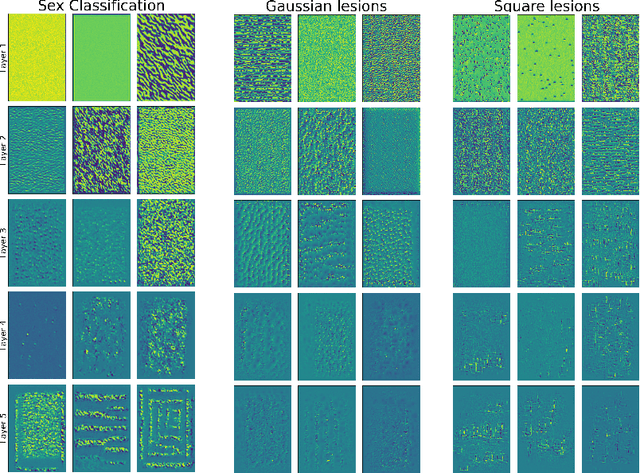
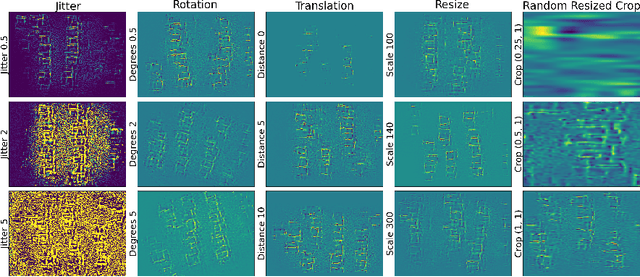
Abstract:A major prerequisite for the application of machine learning models in clinical decision making is trust and interpretability. Current explainability studies in the neuroimaging community have mostly focused on explaining individual decisions of trained models, e.g. obtained by a convolutional neural network (CNN). Using attribution methods such as layer-wise relevance propagation or SHAP heatmaps can be created that highlight which regions of an input are more relevant for the decision than others. While this allows the detection of potential data set biases and can be used as a guide for a human expert, it does not allow an understanding of the underlying principles the model has learned. In this study, we instead show, to the best of our knowledge, for the first time results using feature visualization of neuroimaging CNNs. Particularly, we have trained CNNs for different tasks including sex classification and artificial lesion classification based on structural magnetic resonance imaging (MRI) data. We have then iteratively generated images that maximally activate specific neurons, in order to visualize the patterns they respond to. To improve the visualizations we compared several regularization strategies. The resulting images reveal the learned concepts of the artificial lesions, including their shapes, but remain hard to interpret for abstract features in the sex classification task.
Evaluating saliency methods on artificial data with different background types
Dec 09, 2021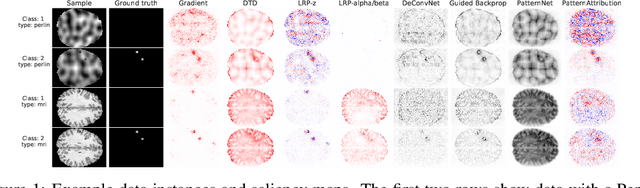
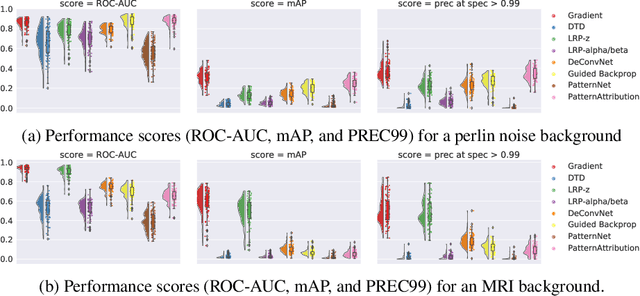
Abstract:Over the last years, many 'explainable artificial intelligence' (xAI) approaches have been developed, but these have not always been objectively evaluated. To evaluate the quality of heatmaps generated by various saliency methods, we developed a framework to generate artificial data with synthetic lesions and a known ground truth map. Using this framework, we evaluated two data sets with different backgrounds, Perlin noise and 2D brain MRI slices, and found that the heatmaps vary strongly between saliency methods and backgrounds. We strongly encourage further evaluation of saliency maps and xAI methods using this framework before applying these in clinical or other safety-critical settings.
Harnessing spatial homogeneity of neuroimaging data: patch individual filter layers for CNNs
Jul 23, 2020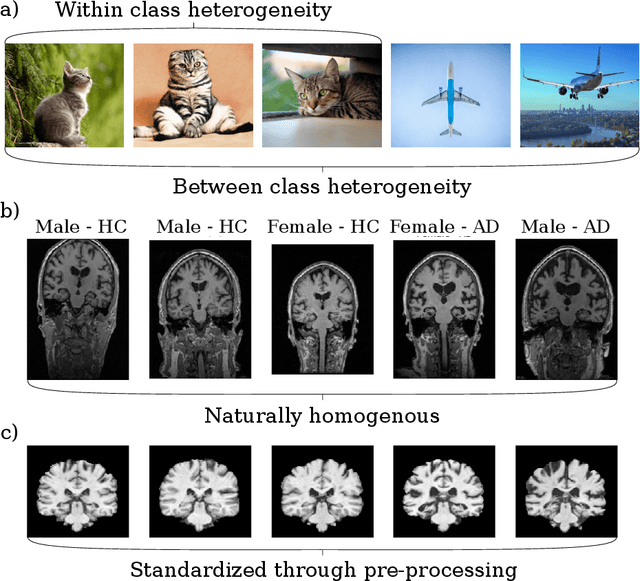
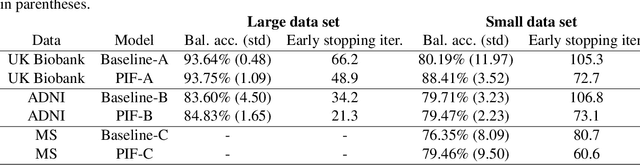
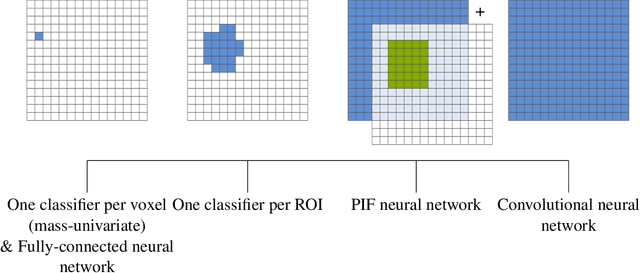
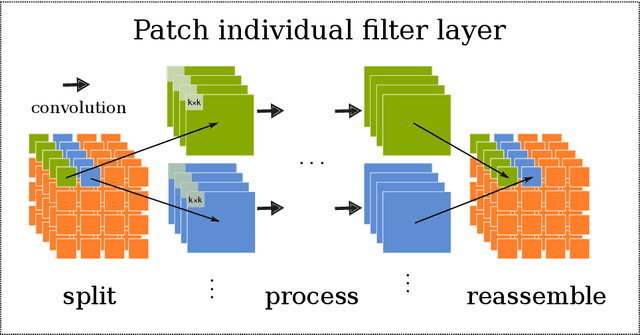
Abstract:Neuroimaging data, e.g. obtained from magnetic resonance imaging (MRI), is comparably homogeneous due to (1) the uniform structure of the brain and (2) additional efforts to spatially normalize the data to a standard template using linear and non-linear transformations. Convolutional neural networks (CNNs), in contrast, have been specifically designed for highly heterogeneous data, such as natural images, by sliding convolutional filters over different positions in an image. Here, we suggest a new CNN architecture that combines the idea of hierarchical abstraction in neural networks with a prior on the spatial homogeneity of neuroimaging data: Whereas early layers are trained globally using standard convolutional layers, we introduce for higher, more abstract layers patch individual filters (PIF). By learning filters in individual image regions (patches) without sharing weights, PIF layers can learn abstract features faster and with fewer samples. We thoroughly evaluated PIF layers for three different tasks and data sets, namely sex classification on UK Biobank data, Alzheimer's disease detection on ADNI data and multiple sclerosis detection on private hospital data. We demonstrate that CNNs using PIF layers result in higher accuracies, especially in low sample size settings, and need fewer training epochs for convergence. To the best of our knowledge, this is the first study which introduces a prior on brain MRI for CNN learning.
Harnessing spatial MRI normalization: patch individual filter layers for CNNs
Nov 14, 2019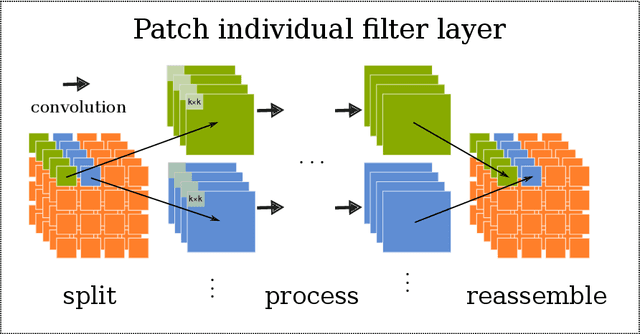
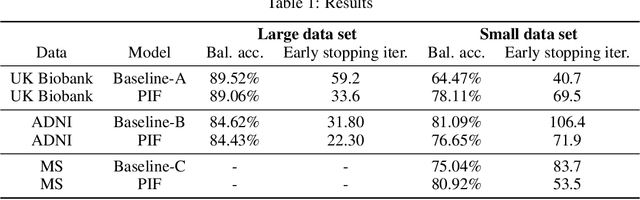
Abstract:Neuroimaging studies based on magnetic resonance imaging (MRI) typically employ rigorous forms of preprocessing. Images are spatially normalized to a standard template using linear and non-linear transformations. Thus, one can assume that a patch at location (x, y, height, width) contains the same brain region across the entire data set. Most analyses applied on brain MRI using convolutional neural networks (CNNs) ignore this distinction from natural images. Here, we suggest a new layer type called patch individual filter (PIF) layer, which trains higher-level filters locally as we assume that more abstract features are locally specific after spatial normalization. We evaluate PIF layers on three different tasks, namely sex classification as well as either Alzheimer's disease (AD) or multiple sclerosis (MS) detection. We demonstrate that CNNs using PIF layers outperform their counterparts in several, especially low sample size settings.
Testing the robustness of attribution methods for convolutional neural networks in MRI-based Alzheimer's disease classification
Sep 19, 2019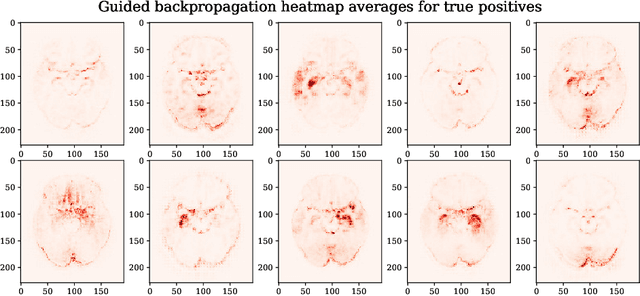
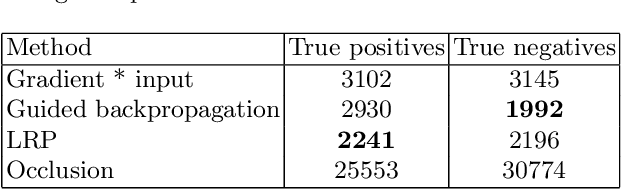
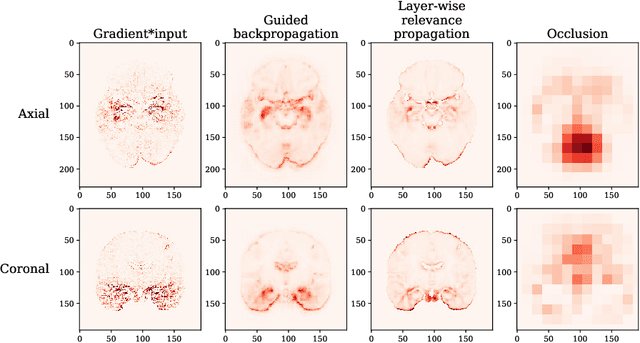

Abstract:Attribution methods are an easy to use tool for investigating and validating machine learning models. Multiple methods have been suggested in the literature and it is not yet clear which method is most suitable for a given task. In this study, we tested the robustness of four attribution methods, namely gradient*input, guided backpropagation, layer-wise relevance propagation and occlusion, for the task of Alzheimer's disease classification. We have repeatedly trained a convolutional neural network (CNN) with identical training settings in order to separate structural MRI data of patients with Alzheimer's disease and healthy controls. Afterwards, we produced attribution maps for each subject in the test data and quantitatively compared them across models and attribution methods. We show that visual comparison is not sufficient and that some widely used attribution methods produce highly inconsistent outcomes.
Uncovering convolutional neural network decisions for diagnosing multiple sclerosis on conventional MRI using layer-wise relevance propagation
Apr 18, 2019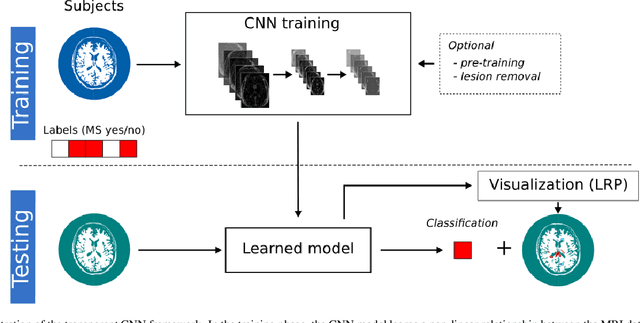
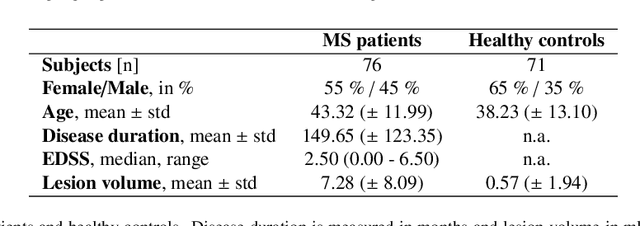
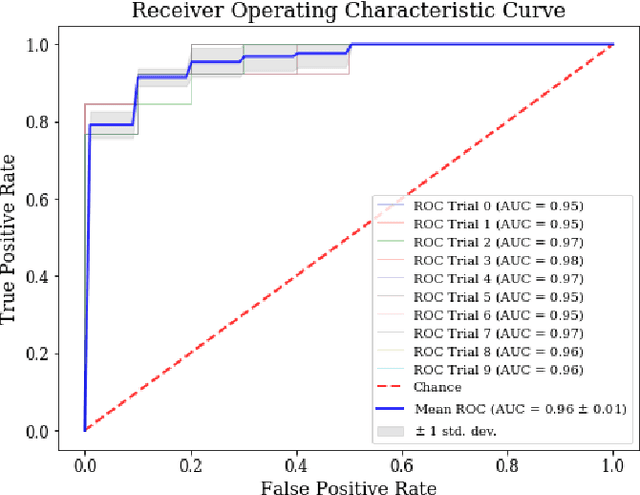
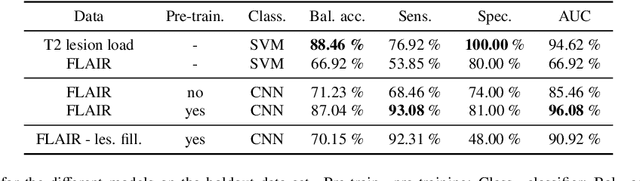
Abstract:Machine learning-based imaging diagnostics has recently reached or even superseded the level of clinical experts in several clinical domains. However, classification decisions of a trained machine learning system are typically non-transparent, a major hindrance for clinical integration, error tracking or knowledge discovery. In this study, we present a transparent deep learning framework relying on convolutional neural networks (CNNs) and layer-wise relevance propagation (LRP) for diagnosing multiple sclerosis (MS). MS is commonly diagnosed utilizing a combination of clinical presentation and conventional magnetic resonance imaging (MRI), specifically the occurrence and presentation of white matter lesions in T2-weighted images. We hypothesized that using LRP in a naive predictive model would enable us to uncover relevant image features that a trained CNN uses for decision-making. Since imaging markers in MS are well-established this would enable us to validate the respective CNN model. First, we pre-trained a CNN on MRI data from the Alzheimer's Disease Neuroimaging Initiative (n = 921), afterwards specializing the CNN to discriminate between MS patients and healthy controls (n = 147). Using LRP, we then produced a heatmap for each subject in the holdout set depicting the voxel-wise relevance for a particular classification decision. The resulting CNN model resulted in a balanced accuracy of 87.04% and an area under the curve of 96.08% in a receiver operating characteristic curve. The subsequent LRP visualization revealed that the CNN model focuses indeed on individual lesions, but also incorporates additional information such as lesion location, non-lesional white matter or gray matter areas such as the thalamus, which are established conventional and advanced MRI markers in MS. We conclude that LRP and the proposed framework have the capability to make diagnostic decisions of...
Visualizing Convolutional Networks for MRI-based Diagnosis of Alzheimer's Disease
Aug 08, 2018


Abstract:Visualizing and interpreting convolutional neural networks (CNNs) is an important task to increase trust in automatic medical decision making systems. In this study, we train a 3D CNN to detect Alzheimer's disease based on structural MRI scans of the brain. Then, we apply four different gradient-based and occlusion-based visualization methods that explain the network's classification decisions by highlighting relevant areas in the input image. We compare the methods qualitatively and quantitatively. We find that all four methods focus on brain regions known to be involved in Alzheimer's disease, such as inferior and middle temporal gyrus. While the occlusion-based methods focus more on specific regions, the gradient-based methods pick up distributed relevance patterns. Additionally, we find that the distribution of relevance varies across patients, with some having a stronger focus on the temporal lobe, whereas for others more cortical areas are relevant. In summary, we show that applying different visualization methods is important to understand the decisions of a CNN, a step that is crucial to increase clinical impact and trust in computer-based decision support systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge