Ewout W. Steyerberg
topic group 6 of the STRATOS initiative
Performance evaluation of predictive AI models to support medical decisions: Overview and guidance
Dec 13, 2024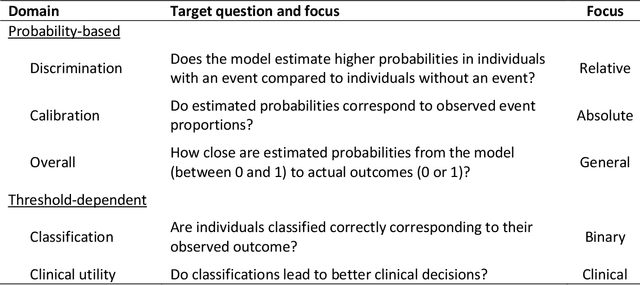
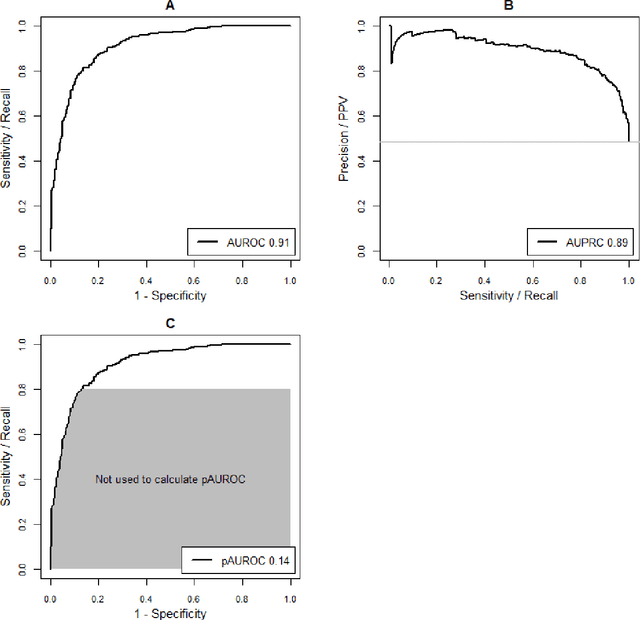
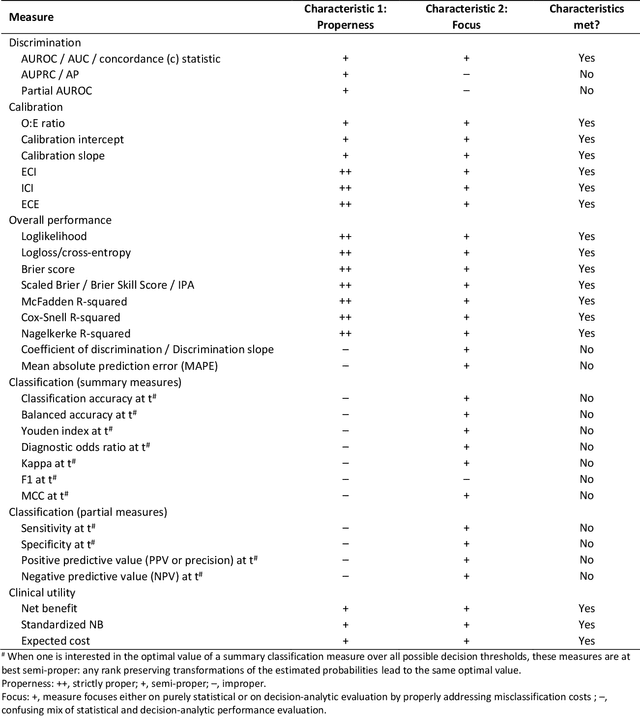
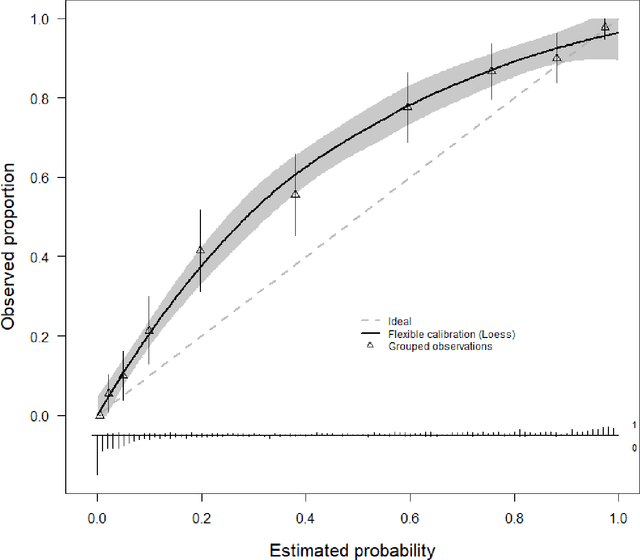
Abstract:A myriad of measures to illustrate performance of predictive artificial intelligence (AI) models have been proposed in the literature. Selecting appropriate performance measures is essential for predictive AI models that are developed to be used in medical practice, because poorly performing models may harm patients and lead to increased costs. We aim to assess the merits of classic and contemporary performance measures when validating predictive AI models for use in medical practice. We focus on models with a binary outcome. We discuss 32 performance measures covering five performance domains (discrimination, calibration, overall, classification, and clinical utility) along with accompanying graphical assessments. The first four domains cover statistical performance, the fifth domain covers decision-analytic performance. We explain why two key characteristics are important when selecting which performance measures to assess: (1) whether the measure's expected value is optimized when it is calculated using the correct probabilities (i.e., a "proper" measure), and (2) whether they reflect either purely statistical performance or decision-analytic performance by properly considering misclassification costs. Seventeen measures exhibit both characteristics, fourteen measures exhibited one characteristic, and one measure possessed neither characteristic (the F1 measure). All classification measures (such as classification accuracy and F1) are improper for clinically relevant decision thresholds other than 0.5 or the prevalence. We recommend the following measures and plots as essential to report: AUROC, calibration plot, a clinical utility measure such as net benefit with decision curve analysis, and a plot with probability distributions per outcome category.
The leap to ordinal: functional prognosis after traumatic brain injury using artificial intelligence
Feb 10, 2022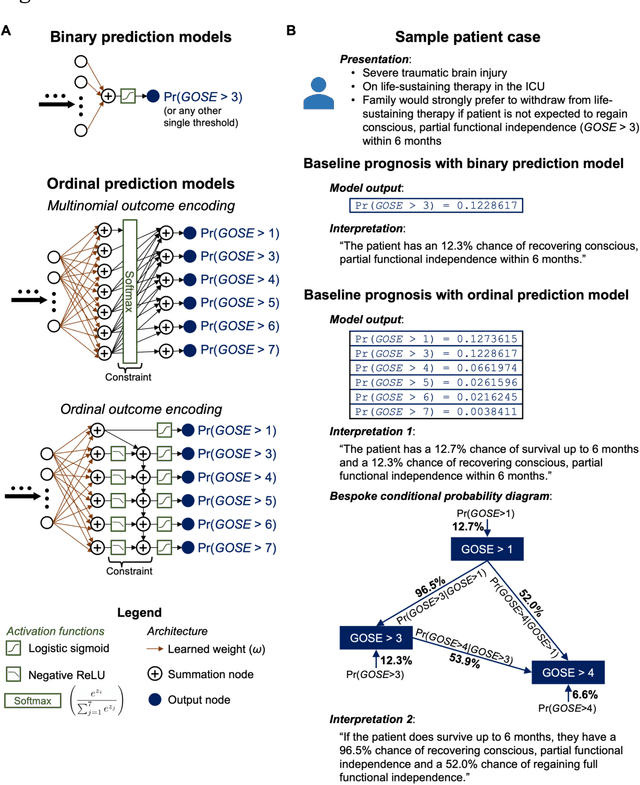
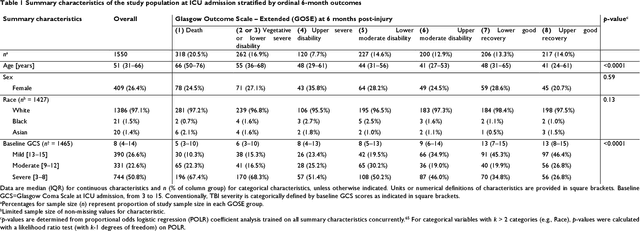
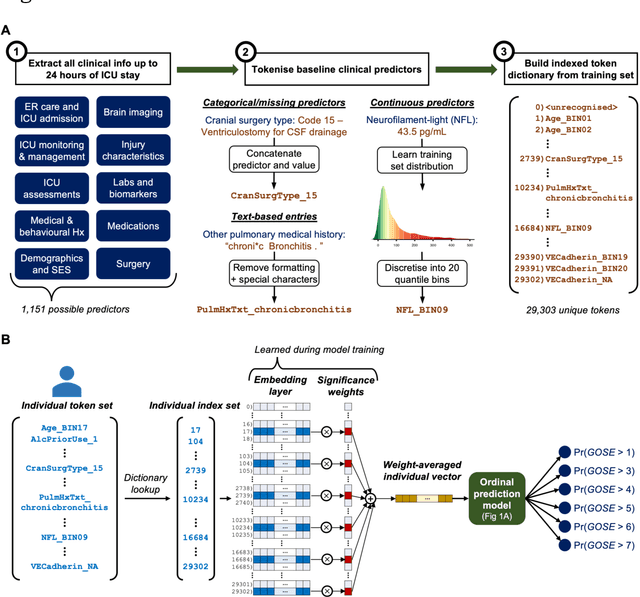
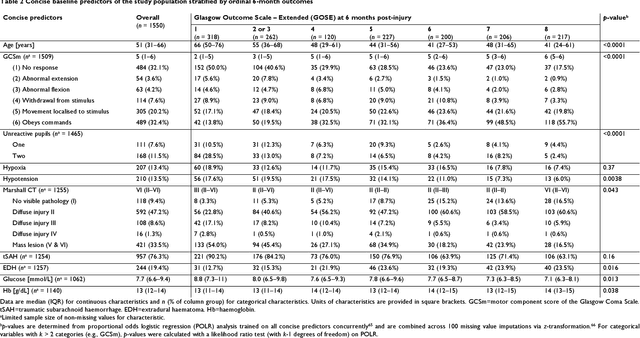
Abstract:When a patient is admitted to the intensive care unit (ICU) after a traumatic brain injury (TBI), an early prognosis is essential for baseline risk adjustment and shared decision making. TBI outcomes are commonly categorised by the Glasgow Outcome Scale-Extended (GOSE) into 8, ordered levels of functional recovery at 6 months after injury. Existing ICU prognostic models predict binary outcomes at a certain threshold of GOSE (e.g., prediction of survival [GOSE>1] or functional independence [GOSE>4]). We aimed to develop ordinal prediction models that concurrently predict probabilities of each GOSE score. From a prospective cohort (n=1,550, 65 centres) in the ICU stratum of the Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) patient dataset, we extracted all clinical information within 24 hours of ICU admission (1,151 predictors) and 6-month GOSE scores. We analysed the effect of 2 design elements on ordinal model performance: (1) the baseline predictor set, ranging from a concise set of 10 validated predictors to a token-embedded representation of all possible predictors, and (2) the modelling strategy, from ordinal logistic regression to multinomial deep learning. With repeated k-fold cross-validation, we found that expanding the baseline predictor set significantly improved ordinal prediction performance while increasing analytical complexity did not. Half of these gains could be achieved with the addition of 8 high-impact predictors (2 demographic variables, 4 protein biomarkers, and 2 severity assessments) to the concise set. At best, ordinal models achieved 0.76 (95% CI: 0.74-0.77) ordinal discrimination ability (ordinal c-index) and 57% (95% CI: 54%-60%) explanation of ordinal variation in 6-month GOSE (Somers' D). Our results motivate the search for informative predictors for higher GOSE and the development of ordinal dynamic prediction models.
A standardized framework for risk-based assessment of treatment effect heterogeneity in observational healthcare databases
Oct 13, 2020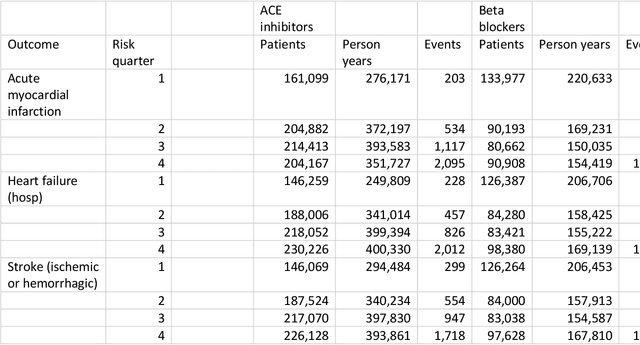
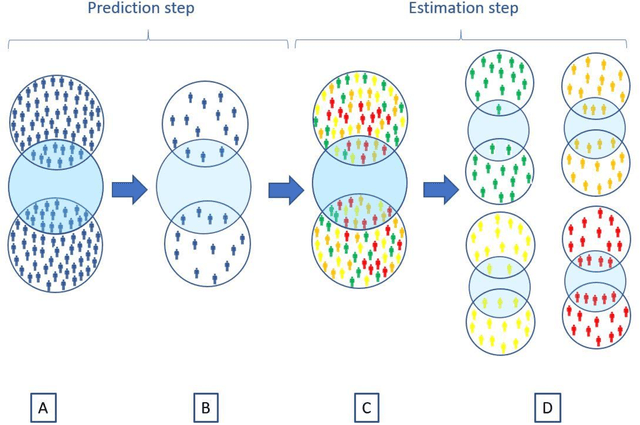
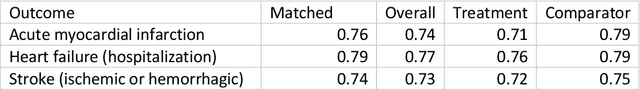
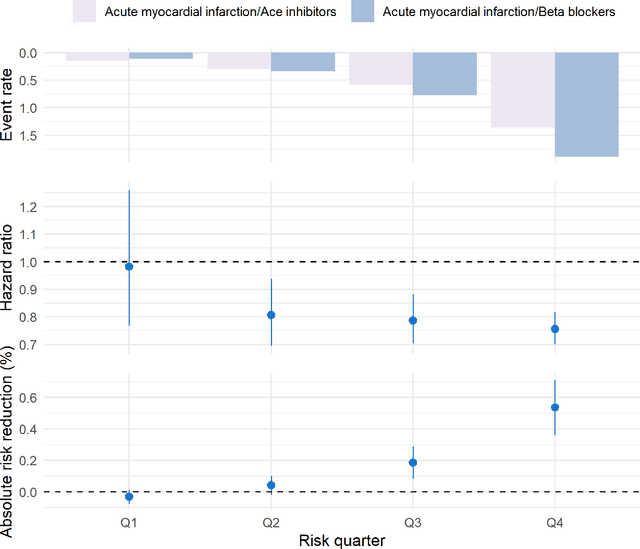
Abstract:Aim: One of the aims of the Observation Health Data Sciences and Informatics (OHDSI) initiative is population-level treatment effect estimation in large observational databases. Since treatment effects are well-known to vary across groups of patients with different baseline risk, we aimed to extend the OHDSI methods library with a framework for risk-based assessment of treatment effect heterogeneity. Materials and Methods: The proposed framework consists of five steps: 1) definition of the problem, i.e. the population, the treatment, the comparator and the outcome(s) of interest; 2) identification of relevant databases; 3) development of a prediction model for the outcome(s) of interest; 4) estimation of propensity scores within strata of predicted risk and estimation of relative and absolute treatment effect within strata of predicted risk; 5) evaluation and presentation of results. Results: We demonstrate our framework by evaluating heterogeneity of the effect of angiotensin-converting enzyme (ACE) inhibitors versus beta blockers on a set of 9 outcomes of interest across three observational databases. With increasing risk of acute myocardial infarction we observed increasing absolute benefits, i.e. from -0.03% to 0.54% in the lowest to highest risk groups. Cough-related absolute harms decreased from 4.1% to 2.6%. Conclusions: The proposed framework may be useful for the evaluation of heterogeneity of treatment effect on observational data that are mapped to the OMOP Common Data Model. The proof of concept study demonstrates its feasibility in large observational data. Further insights may arise by application to safety and effectiveness questions across the global data network.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge