Lindsay Wilson
Contribution of clinical course to outcome after traumatic brain injury: mining patient trajectories from European intensive care unit data
Mar 08, 2023Abstract:Existing methods to characterise the evolving condition of traumatic brain injury (TBI) patients in the intensive care unit (ICU) do not capture the context necessary for individualising treatment. We aimed to develop a modelling strategy which integrates all data stored in medical records to produce an interpretable disease course for each TBI patient's ICU stay. From a prospective, European cohort (n=1,550, 65 centres, 19 countries) of TBI patients, we extracted all 1,166 variables collected before or during ICU stay as well as 6-month functional outcome on the Glasgow Outcome Scale-Extended (GOSE). We trained recurrent neural network models to map a token-embedded time series representation of all variables (including missing data) to an ordinal GOSE prognosis every 2 hours. With repeated cross-validation, we evaluated calibration and the explanation of ordinal variance in GOSE with Somers' Dxy. Furthermore, we applied TimeSHAP to calculate the contribution of variables and prior timepoints towards transitions in patient trajectories. Our modelling strategy achieved calibration at 8 hours, and the full range of variables explained up to 52% (95% CI: 50-54%) of the variance in ordinal functional outcome. Up to 91% (90-91%) of this explanation was derived from pre-ICU and admission information. Information collected in the ICU increased explanation (by up to 5% [4-6%]), though not enough to counter poorer performance in longer-stay (>5.75 days) patients. Static variables with the highest contributions were physician prognoses and certain demographic and CT features. Among dynamic variables, markers of intracranial hypertension and neurological function contributed the most. Whilst static information currently accounts for the majority of functional outcome explanation, our data-driven analysis highlights investigative avenues to improve dynamic characterisation of longer-stay patients.
The leap to ordinal: functional prognosis after traumatic brain injury using artificial intelligence
Feb 10, 2022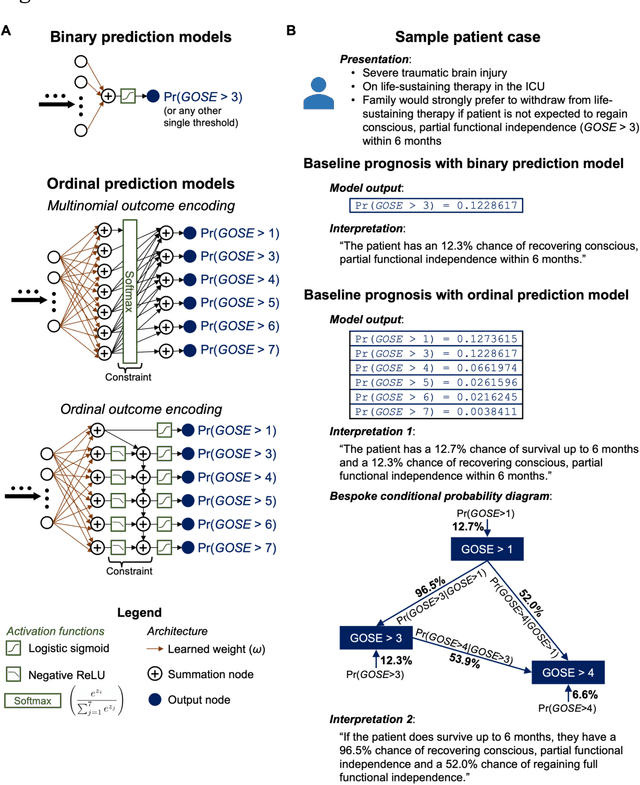
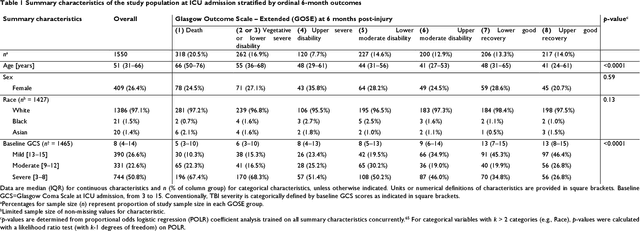
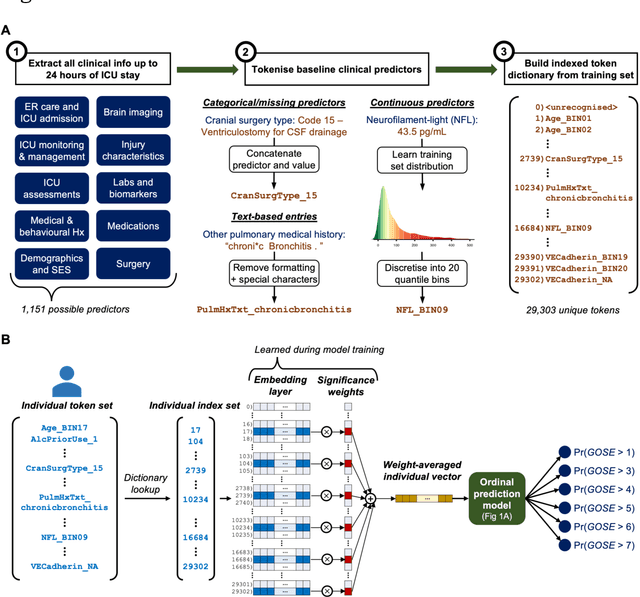
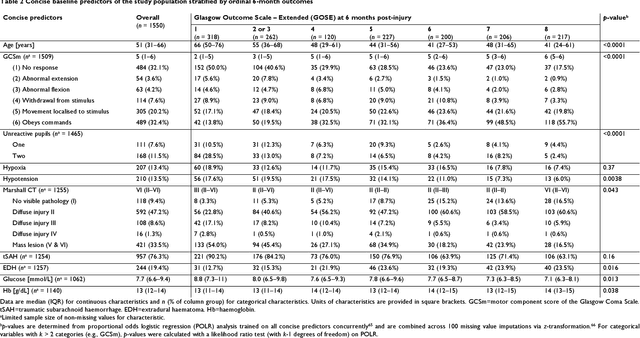
Abstract:When a patient is admitted to the intensive care unit (ICU) after a traumatic brain injury (TBI), an early prognosis is essential for baseline risk adjustment and shared decision making. TBI outcomes are commonly categorised by the Glasgow Outcome Scale-Extended (GOSE) into 8, ordered levels of functional recovery at 6 months after injury. Existing ICU prognostic models predict binary outcomes at a certain threshold of GOSE (e.g., prediction of survival [GOSE>1] or functional independence [GOSE>4]). We aimed to develop ordinal prediction models that concurrently predict probabilities of each GOSE score. From a prospective cohort (n=1,550, 65 centres) in the ICU stratum of the Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) patient dataset, we extracted all clinical information within 24 hours of ICU admission (1,151 predictors) and 6-month GOSE scores. We analysed the effect of 2 design elements on ordinal model performance: (1) the baseline predictor set, ranging from a concise set of 10 validated predictors to a token-embedded representation of all possible predictors, and (2) the modelling strategy, from ordinal logistic regression to multinomial deep learning. With repeated k-fold cross-validation, we found that expanding the baseline predictor set significantly improved ordinal prediction performance while increasing analytical complexity did not. Half of these gains could be achieved with the addition of 8 high-impact predictors (2 demographic variables, 4 protein biomarkers, and 2 severity assessments) to the concise set. At best, ordinal models achieved 0.76 (95% CI: 0.74-0.77) ordinal discrimination ability (ordinal c-index) and 57% (95% CI: 54%-60%) explanation of ordinal variation in 6-month GOSE (Somers' D). Our results motivate the search for informative predictors for higher GOSE and the development of ordinal dynamic prediction models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge