Esther Puyol Anton
Invisible Attributes, Visible Biases: Exploring Demographic Shortcuts in MRI-based Alzheimer's Disease Classification
Sep 11, 2025Abstract:Magnetic resonance imaging (MRI) is the gold standard for brain imaging. Deep learning (DL) algorithms have been proposed to aid in the diagnosis of diseases such as Alzheimer's disease (AD) from MRI scans. However, DL algorithms can suffer from shortcut learning, in which spurious features, not directly related to the output label, are used for prediction. When these features are related to protected attributes, they can lead to performance bias against underrepresented protected groups, such as those defined by race and sex. In this work, we explore the potential for shortcut learning and demographic bias in DL based AD diagnosis from MRI. We first investigate if DL algorithms can identify race or sex from 3D brain MRI scans to establish the presence or otherwise of race and sex based distributional shifts. Next, we investigate whether training set imbalance by race or sex can cause a drop in model performance, indicating shortcut learning and bias. Finally, we conduct a quantitative and qualitative analysis of feature attributions in different brain regions for both the protected attribute and AD classification tasks. Through these experiments, and using multiple datasets and DL models (ResNet and SwinTransformer), we demonstrate the existence of both race and sex based shortcut learning and bias in DL based AD classification. Our work lays the foundation for fairer DL diagnostic tools in brain MRI. The code is provided at https://github.com/acharaakshit/ShortMR
Automated quantification of myocardial tissue characteristics from native T1 mapping using neural networks with Bayesian inference for uncertainty-based quality-control
Jan 31, 2020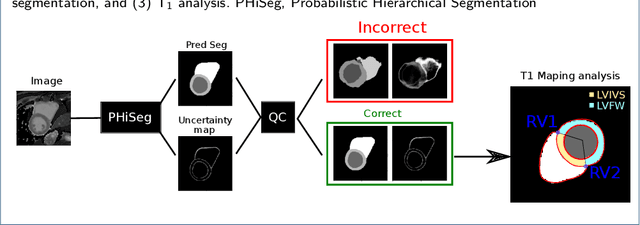
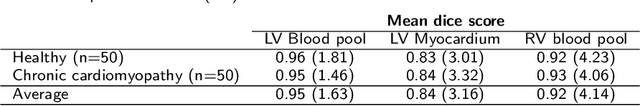
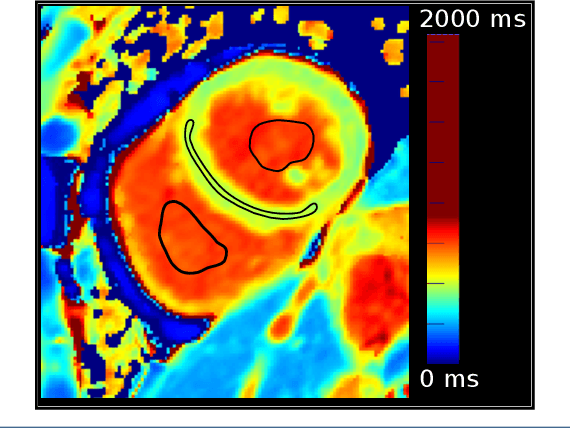

Abstract:Tissue characterisation with CMR parametric mapping has the potential to detect and quantify both focal and diffuse alterations in myocardial structure not assessable by late gadolinium enhancement. Native T1 mapping in particular has shown promise as a useful biomarker to support diagnostic, therapeutic and prognostic decision-making in ischaemic and non-ischaemic cardiomyopathies. Convolutional neural networks with Bayesian inference are a category of artificial neural networks which model the uncertainty of the network output. This study presents an automated framework for tissue characterisation from native ShMOLLI T1 mapping at 1.5T using a Probabilistic Hierarchical Segmentation (PHiSeg) network. In addition, we use the uncertainty information provided by the PHiSeg network in a novel automated quality control (QC) step to identify uncertain T1 values. The PHiSeg network and QC were validated against manual analysis on a cohort of the UK Biobank containing healthy subjects and chronic cardiomyopathy patients. We used the proposed method to obtain reference T1 ranges for the left ventricular myocardium in healthy subjects as well as common clinical cardiac conditions. T1 values computed from automatic and manual segmentations were highly correlated (r=0.97). Bland-Altman analysis showed good agreement between the automated and manual measurements. The average Dice metric was 0.84 for the left ventricular myocardium. The sensitivity of detection of erroneous outputs was 91%. Finally, T1 values were automatically derived from 14,683 CMR exams from the UK Biobank. The proposed pipeline allows for automatic analysis of myocardial native T1 mapping and includes a QC process to detect potentially erroneous results. T1 reference values were presented for healthy subjects and common clinical cardiac conditions from the largest cohort to date using T1-mapping images.
Deep Learning Based Detection and Correction of Cardiac MR Motion Artefacts During Reconstruction for High-Quality Segmentation
Oct 21, 2019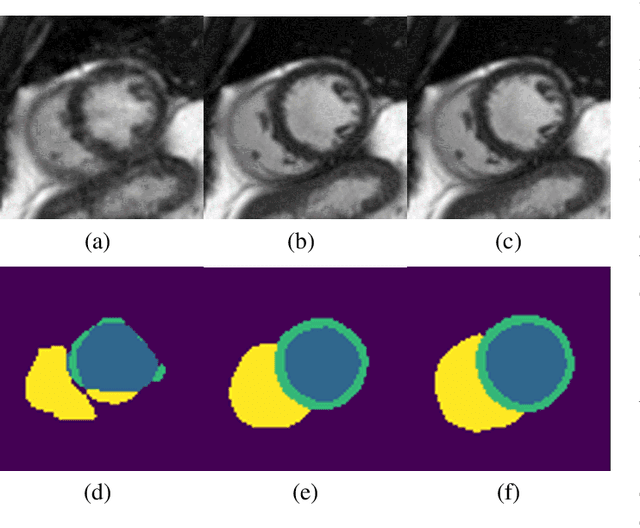
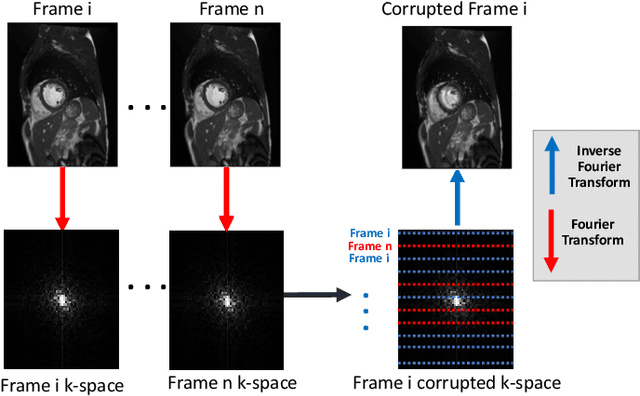
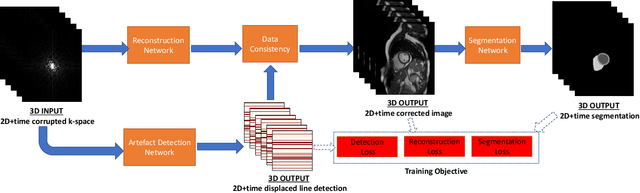
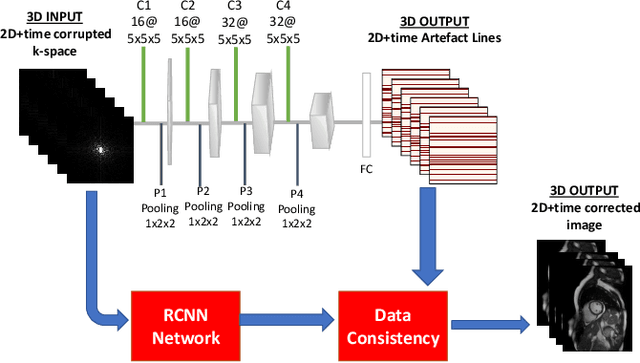
Abstract:Segmenting anatomical structures in medical images has been successfully addressed with deep learning methods for a range of applications. However, this success is heavily dependent on the quality of the image that is being segmented. A commonly neglected point in the medical image analysis community is the vast amount of clinical images that have severe image artefacts due to organ motion, movement of the patient and/or image acquisition related issues. In this paper, we discuss the implications of image motion artefacts on cardiac MR segmentation and compare a variety of approaches for jointly correcting for artefacts and segmenting the cardiac cavity. We propose to use a segmentation network coupled with this in an end-to-end framework. Our training optimises three different tasks: 1) image artefact detection, 2) artefact correction and 3) image segmentation. We train the reconstruction network to automatically correct for motion-related artefacts using synthetically corrupted cardiac MR k-space data and uncorrected reconstructed images. Using a test set of 500 2D+time cine MR acquisitions from the UK Biobank data set, we achieve demonstrably good image quality and high segmentation accuracy in the presence of synthetic motion artefacts. We quantitatively compare our method with a variety of techniques for jointly recovering image quality and performing image segmentation. We showcase better performance compared to state-of-the-art image correction techniques. Moreover, our method preserves the quality of uncorrupted images and therefore can be utilised as a global image reconstruction algorithm.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge