Elina Kontio
Recommender Engine Driven Client Selection in Federated Brain Tumor Segmentation
Dec 28, 2024



Abstract:This study presents a robust and efficient client selection protocol designed to optimize the Federated Learning (FL) process for the Federated Tumor Segmentation Challenge (FeTS 2024). In the evolving landscape of FL, the judicious selection of collaborators emerges as a critical determinant for the success and efficiency of collective learning endeavors, particularly in domains requiring high precision. This work introduces a recommender engine framework based on non-negative matrix factorization (NNMF) and a hybrid aggregation approach that blends content-based and collaborative filtering. This method intelligently analyzes historical performance, expertise, and other relevant metrics to identify the most suitable collaborators. This approach not only addresses the cold start problem where new or inactive collaborators pose selection challenges due to limited data but also significantly improves the precision and efficiency of the FL process. Additionally, we propose harmonic similarity weight aggregation (HSimAgg) for adaptive aggregation of model parameters. We utilized a dataset comprising 1,251 multi-parametric magnetic resonance imaging (mpMRI) scans from individuals diagnosed with glioblastoma (GBM) for training purposes and an additional 219 mpMRI scans for external evaluations. Our federated tumor segmentation approach achieved dice scores of 0.7298, 0.7424, and 0.8218 for enhancing tumor (ET), tumor core (TC), and whole tumor (WT) segmentation tasks respectively on the external validation set. In conclusion, this research demonstrates that selecting collaborators with expertise aligned to specific tasks, like brain tumor segmentation, improves the effectiveness of FL networks.
Election of Collaborators via Reinforcement Learning for Federated Brain Tumor Segmentation
Dec 28, 2024


Abstract:Federated learning (FL) enables collaborative model training across decentralized datasets while preserving data privacy. However, optimally selecting participating collaborators in dynamic FL environments remains challenging. We present RL-HSimAgg, a novel reinforcement learning (RL) and similarity-weighted aggregation (simAgg) algorithm using harmonic mean to manage outlier data points. This paper proposes applying multi-armed bandit algorithms to improve collaborator selection and model generalization. By balancing exploration-exploitation trade-offs, these RL methods can promote resource-efficient training with diverse datasets. We demonstrate the effectiveness of Epsilon-greedy (EG) and upper confidence bound (UCB) algorithms for federated brain lesion segmentation. In simulation experiments on internal and external validation sets, RL-HSimAgg with UCB collaborator outperformed the EG method across all metrics, achieving higher Dice scores for Enhancing Tumor (0.7334 vs 0.6797), Tumor Core (0.7432 vs 0.6821), and Whole Tumor (0.8252 vs 0.7931) segmentation. Therefore, for the Federated Tumor Segmentation Challenge (FeTS 2024), we consider UCB as our primary client selection approach in federated Glioblastoma lesion segmentation of multi-modal MRIs. In conclusion, our research demonstrates that RL-based collaborator management, e.g. using UCB, can potentially improve model robustness and flexibility in distributed learning environments, particularly in domains like brain tumor segmentation.
Differential Privacy for Adaptive Weight Aggregation in Federated Tumor Segmentation
Aug 01, 2023


Abstract:Federated Learning (FL) is a distributed machine learning approach that safeguards privacy by creating an impartial global model while respecting the privacy of individual client data. However, the conventional FL method can introduce security risks when dealing with diverse client data, potentially compromising privacy and data integrity. To address these challenges, we present a differential privacy (DP) federated deep learning framework in medical image segmentation. In this paper, we extend our similarity weight aggregation (SimAgg) method to DP-SimAgg algorithm, a differentially private similarity-weighted aggregation algorithm for brain tumor segmentation in multi-modal magnetic resonance imaging (MRI). Our DP-SimAgg method not only enhances model segmentation capabilities but also provides an additional layer of privacy preservation. Extensive benchmarking and evaluation of our framework, with computational performance as a key consideration, demonstrate that DP-SimAgg enables accurate and robust brain tumor segmentation while minimizing communication costs during model training. This advancement is crucial for preserving the privacy of medical image data and safeguarding sensitive information. In conclusion, adding a differential privacy layer in the global weight aggregation phase of the federated brain tumor segmentation provides a promising solution to privacy concerns without compromising segmentation model efficacy. By leveraging DP, we ensure the protection of client data against adversarial attacks and malicious participants.
Regularized Weight Aggregation in Networked Federated Learning for Glioblastoma Segmentation
Jan 30, 2023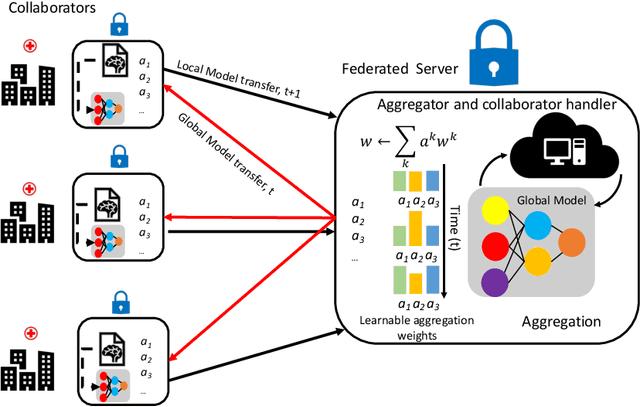

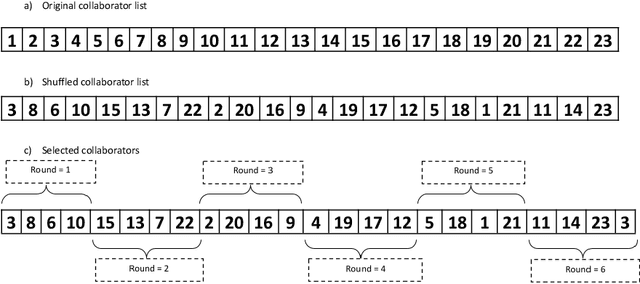
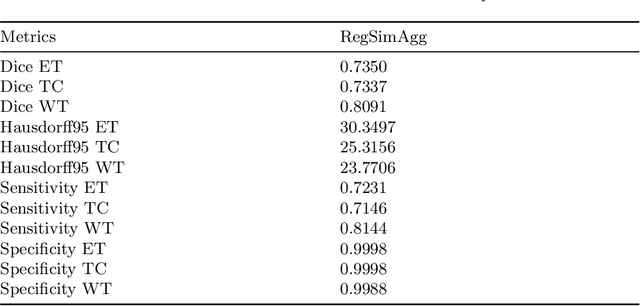
Abstract:In federated learning (FL), the global model at the server requires an efficient mechanism for weight aggregation and a systematic strategy for collaboration selection to manage and optimize communication payload. We introduce a practical and cost-efficient method for regularized weight aggregation and propose a laborsaving technique to select collaborators per round. We illustrate the performance of our method, regularized similarity weight aggregation (RegSimAgg), on the Federated Tumor Segmentation (FeTS) 2022 challenge's federated training (weight aggregation) problem. Our scalable approach is principled, frugal, and suitable for heterogeneous non-IID collaborators. Using FeTS2021 evaluation criterion, our proposed algorithm RegSimAgg stands at 3rd position in the final rankings of FeTS2022 challenge in the weight aggregation task. Our solution is open sourced at: \url{https://github.com/dskhanirfan/FeTS2022}
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge