Elif Ayvali
Trajectory-Optimized Sensing for Active Search of Tissue Abnormalities in Robotic Surgery
May 16, 2018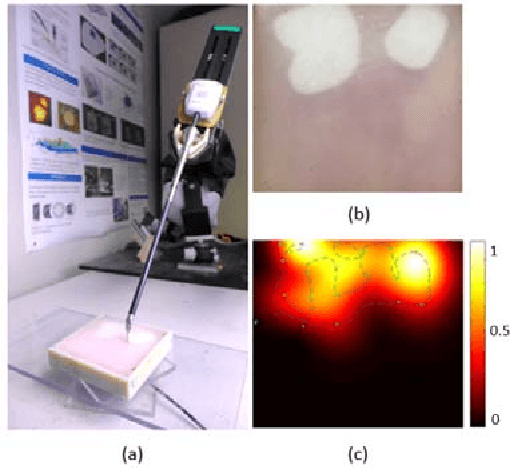
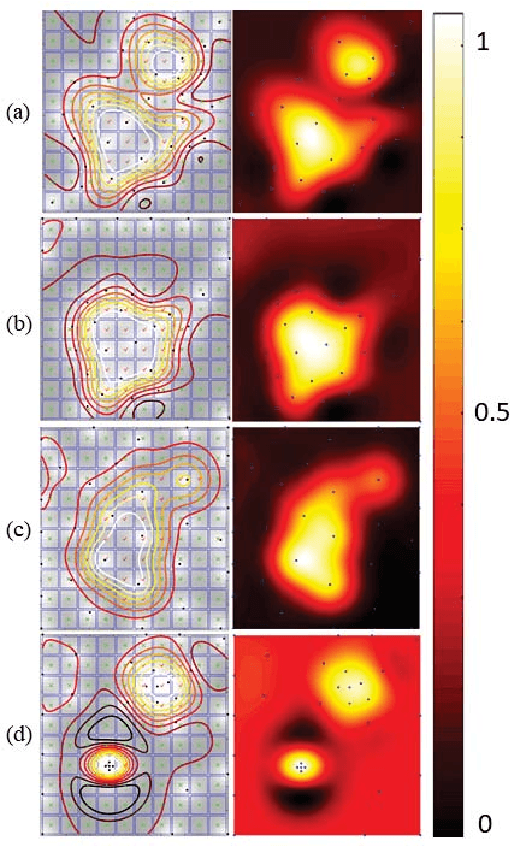
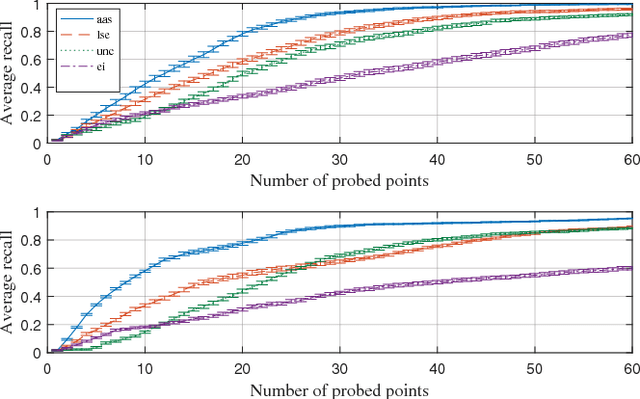
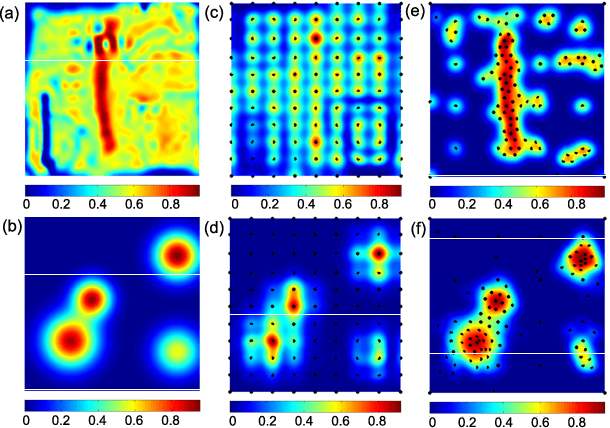
Abstract:In this work, we develop an approach for guiding robots to automatically localize and find the shapes of tumors and other stiff inclusions present in the anatomy. Our approach uses Gaussian processes to model the stiffness distribution and active learning to direct the palpation path of the robot. The palpation paths are chosen such that they maximize an acquisition function provided by an active learning algorithm. Our approach provides the flexibility to avoid obstacles in the robot's path, incorporate uncertainties in robot position and sensor measurements, include prior information about location of stiff inclusions while respecting the robot-kinematics. To the best of our knowledge this is the first work in literature that considers all the above conditions while localizing tumors. The proposed framework is evaluated via simulation and experimentation on three different robot platforms: 6-DoF industrial arm, da Vinci Research Kit (dVRK), and the Insertable Robotic Effector Platform (IREP). Results show that our approach can accurately estimate the locations and boundaries of the stiff inclusions while reducing exploration time.
Ergodic Coverage In Constrained Environments Using Stochastic Trajectory Optimization
Jul 22, 2017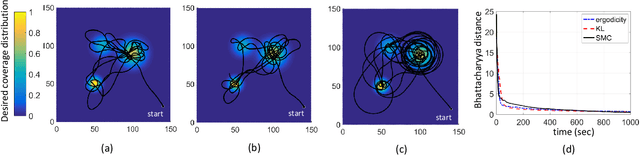
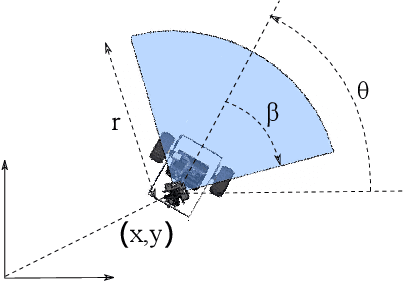
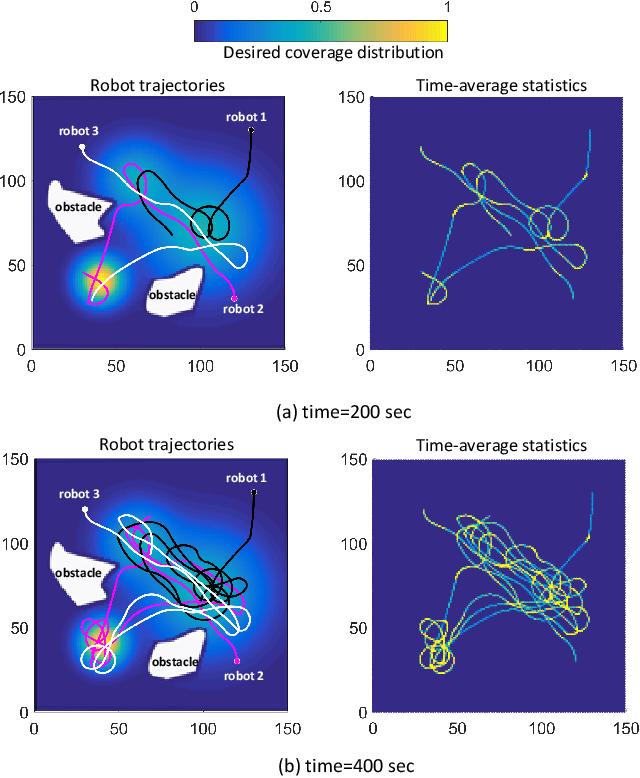
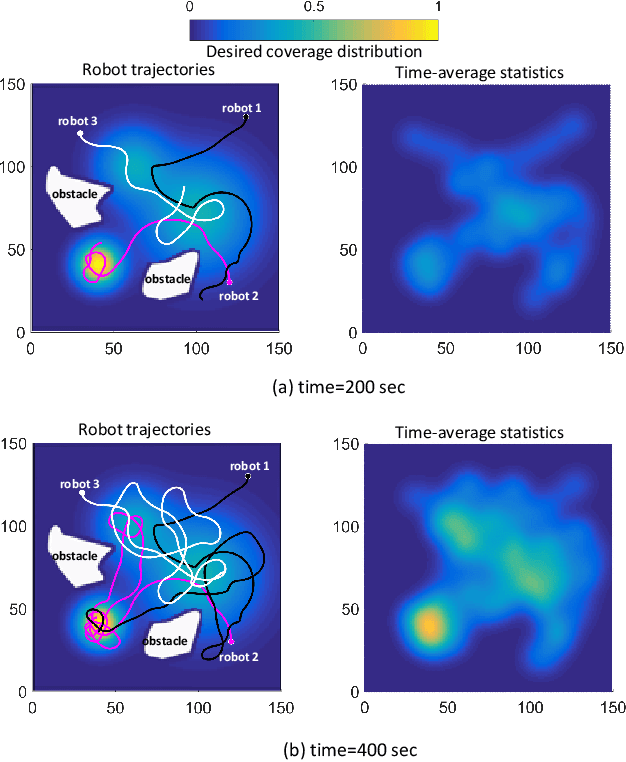
Abstract:In search and surveillance applications in robotics, it is intuitive to spatially distribute robot trajectories with respect to the probability of locating targets in the domain. Ergodic coverage is one such approach to trajectory planning in which a robot is directed such that the percentage of time spent in a region is in proportion to the probability of locating targets in that region. In this work, we extend the ergodic coverage algorithm to robots operating in constrained environments and present a formulation that can capture sensor footprint and avoid obstacles and restricted areas in the domain. We demonstrate that our formulation easily extends to coordination of multiple robots equipped with different sensing capabilities to perform ergodic coverage of a domain.
Using Bayesian Optimization to Guide Probing of a Flexible Environment for Simultaneous Registration and Stiffness Mapping
Sep 19, 2015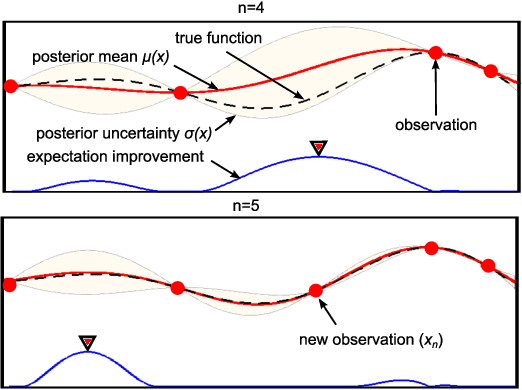
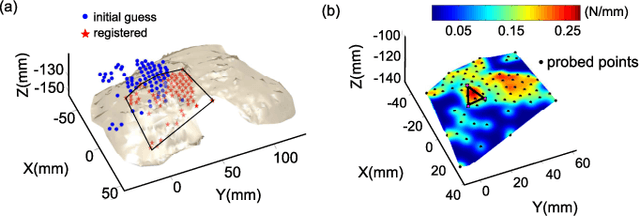
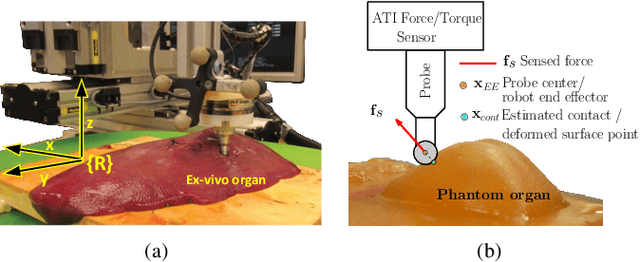
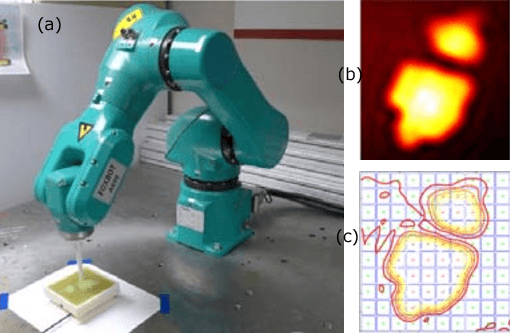
Abstract:One of the goals of computer-aided surgery is to match intraoperative data to preoperative images of the anatomy and add complementary information that can facilitate the task of surgical navigation. In this context, mechanical palpation can reveal critical anatomical features such as arteries and cancerous lumps which are stiffer that the surrounding tissue. This work uses position and force measurements obtained during mechanical palpation for registration and stiffness mapping. Prior approaches, including our own, exhaustively palpated the entire organ to achieve this goal. To overcome the costly palpation of the entire organ, a Bayesian optimization framework is introduced to guide the end effector to palpate stiff regions while simultaneously updating the registration of the end effector to an a priori geometric model of the organ, hence enabling the fusion of ntraoperative data into the a priori model obtained through imaging. This new framework uses Gaussian processes to model the stiffness distribution and Bayesian optimization to direct where to sample next for maximum information gain. The proposed method was evaluated with experimental data obtained using a Cartesian robot interacting with a silicone organ model and an ex vivo porcine liver.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge