Edouard Duchesnay
NEUROSPIN
Robust brain age estimation from structural MRI with contrastive learning
Jul 02, 2025Abstract:Estimating brain age from structural MRI has emerged as a powerful tool for characterizing normative and pathological aging. In this work, we explore contrastive learning as a scalable and robust alternative to supervised approaches for brain age estimation. We introduce a novel contrastive loss function, $\mathcal{L}^{exp}$, and evaluate it across multiple public neuroimaging datasets comprising over 20,000 scans. Our experiments reveal four key findings. First, scaling pre-training on diverse, multi-site data consistently improves generalization performance, cutting external mean absolute error (MAE) nearly in half. Second, $\mathcal{L}^{exp}$ is robust to site-related confounds, maintaining low scanner-predictability as training size increases. Third, contrastive models reliably capture accelerated aging in patients with cognitive impairment and Alzheimer's disease, as shown through brain age gap analysis, ROC curves, and longitudinal trends. Lastly, unlike supervised baselines, $\mathcal{L}^{exp}$ maintains a strong correlation between brain age accuracy and downstream diagnostic performance, supporting its potential as a foundation model for neuroimaging. These results position contrastive learning as a promising direction for building generalizable and clinically meaningful brain representations.
Separating common from salient patterns with Contrastive Representation Learning
Feb 19, 2024Abstract:Contrastive Analysis is a sub-field of Representation Learning that aims at separating common factors of variation between two datasets, a background (i.e., healthy subjects) and a target (i.e., diseased subjects), from the salient factors of variation, only present in the target dataset. Despite their relevance, current models based on Variational Auto-Encoders have shown poor performance in learning semantically-expressive representations. On the other hand, Contrastive Representation Learning has shown tremendous performance leaps in various applications (classification, clustering, etc.). In this work, we propose to leverage the ability of Contrastive Learning to learn semantically expressive representations well adapted for Contrastive Analysis. We reformulate it under the lens of the InfoMax Principle and identify two Mutual Information terms to maximize and one to minimize. We decompose the first two terms into an Alignment and a Uniformity term, as commonly done in Contrastive Learning. Then, we motivate a novel Mutual Information minimization strategy to prevent information leakage between common and salient distributions. We validate our method, called SepCLR, on three visual datasets and three medical datasets, specifically conceived to assess the pattern separation capability in Contrastive Analysis. Code available at https://github.com/neurospin-projects/2024_rlouiset_sep_clr.
SepVAE: a contrastive VAE to separate pathological patterns from healthy ones
Jul 12, 2023Abstract:Contrastive Analysis VAE (CA-VAEs) is a family of Variational auto-encoders (VAEs) that aims at separating the common factors of variation between a background dataset (BG) (i.e., healthy subjects) and a target dataset (TG) (i.e., patients) from the ones that only exist in the target dataset. To do so, these methods separate the latent space into a set of salient features (i.e., proper to the target dataset) and a set of common features (i.e., exist in both datasets). Currently, all models fail to prevent the sharing of information between latent spaces effectively and to capture all salient factors of variation. To this end, we introduce two crucial regularization losses: a disentangling term between common and salient representations and a classification term between background and target samples in the salient space. We show a better performance than previous CA-VAEs methods on three medical applications and a natural images dataset (CelebA). Code and datasets are available on GitHub https://github.com/neurospin-projects/2023_rlouiset_sepvae.
Contrastive learning for regression in multi-site brain age prediction
Nov 14, 2022



Abstract:Building accurate Deep Learning (DL) models for brain age prediction is a very relevant topic in neuroimaging, as it could help better understand neurodegenerative disorders and find new biomarkers. To estimate accurate and generalizable models, large datasets have been collected, which are often multi-site and multi-scanner. This large heterogeneity negatively affects the generalization performance of DL models since they are prone to overfit site-related noise. Recently, contrastive learning approaches have been shown to be more robust against noise in data or labels. For this reason, we propose a novel contrastive learning regression loss for robust brain age prediction using MRI scans. Our method achieves state-of-the-art performance on the OpenBHB challenge, yielding the best generalization capability and robustness to site-related noise.
Rethinking Positive Sampling for Contrastive Learning with Kernel
Jun 03, 2022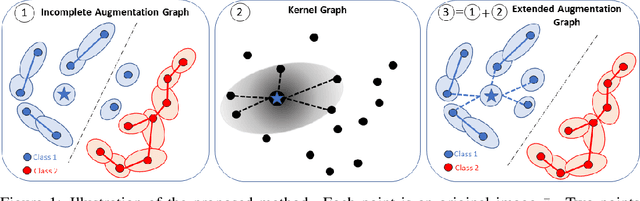
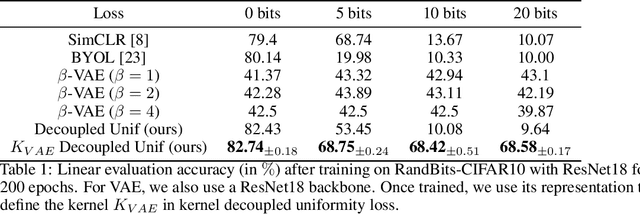
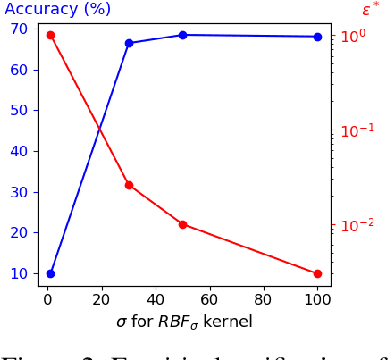
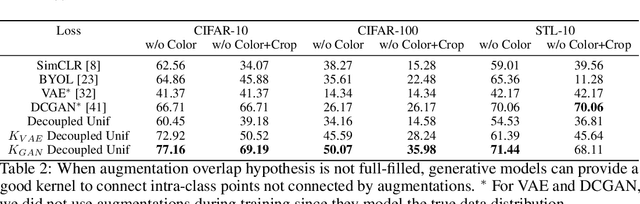
Abstract:Data augmentation is a crucial component in unsupervised contrastive learning (CL). It determines how positive samples are defined and, ultimately, the quality of the representation. While efficient augmentations have been found for standard vision datasets, such as ImageNet, it is still an open problem in other applications, such as medical imaging, or in datasets with easy-to-learn but irrelevant imaging features. In this work, we propose a new way to define positive samples using kernel theory along with a novel loss called decoupled uniformity. We propose to integrate prior information, learnt from generative models or given as auxiliary attributes, into contrastive learning, to make it less dependent on data augmentation. We draw a connection between contrastive learning and the conditional mean embedding theory to derive tight bounds on the downstream classification loss. In an unsupervised setting, we empirically demonstrate that CL benefits from generative models, such as VAE and GAN, to less rely on data augmentations. We validate our framework on vision datasets including CIFAR10, CIFAR100, STL10 and ImageNet100 and a brain MRI dataset. In the weakly supervised setting, we demonstrate that our formulation provides state-of-the-art results.
Conditional Alignment and Uniformity for Contrastive Learning with Continuous Proxy Labels
Nov 10, 2021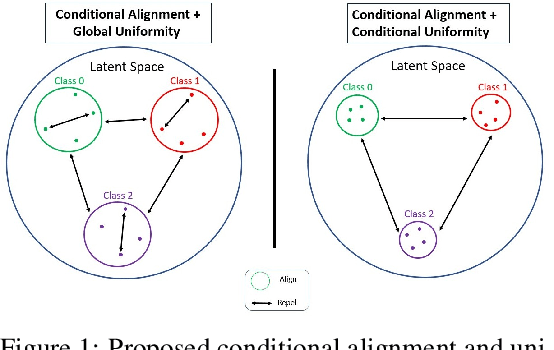
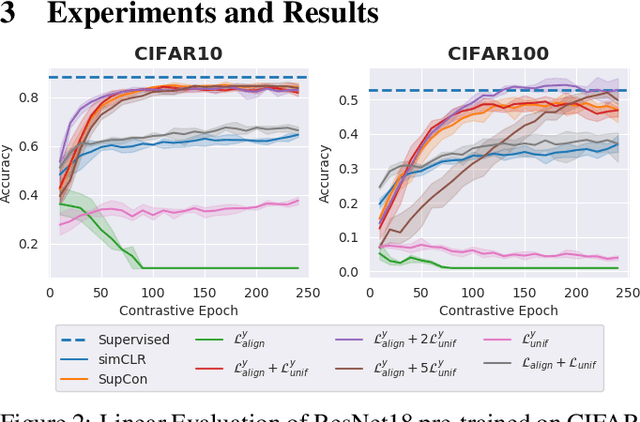
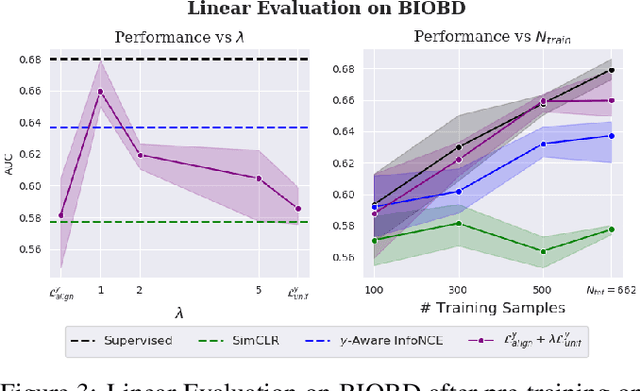
Abstract:Contrastive Learning has shown impressive results on natural and medical images, without requiring annotated data. However, a particularity of medical images is the availability of meta-data (such as age or sex) that can be exploited for learning representations. Here, we show that the recently proposed contrastive y-Aware InfoNCE loss, that integrates multi-dimensional meta-data, asymptotically optimizes two properties: conditional alignment and global uniformity. Similarly to [Wang, 2020], conditional alignment means that similar samples should have similar features, but conditionally on the meta-data. Instead, global uniformity means that the (normalized) features should be uniformly distributed on the unit hyper-sphere, independently of the meta-data. Here, we propose to define conditional uniformity, relying on the meta-data, that repel only samples with dissimilar meta-data. We show that direct optimization of both conditional alignment and uniformity improves the representations, in terms of linear evaluation, on both CIFAR-100 and a brain MRI dataset.
UCSL : A Machine Learning Expectation-Maximization framework for Unsupervised Clustering driven by Supervised Learning
Jul 05, 2021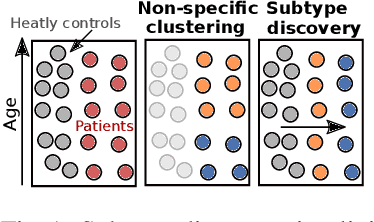
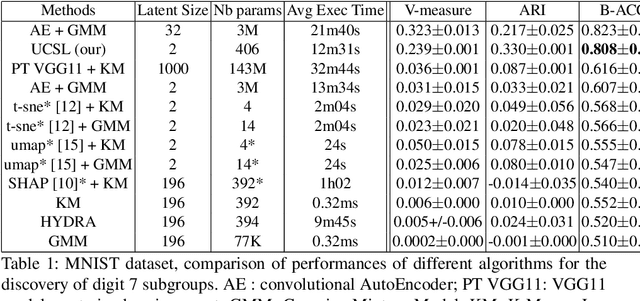
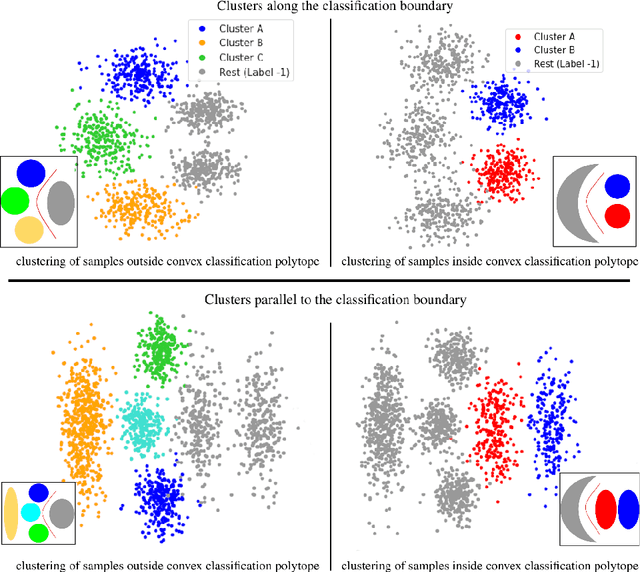
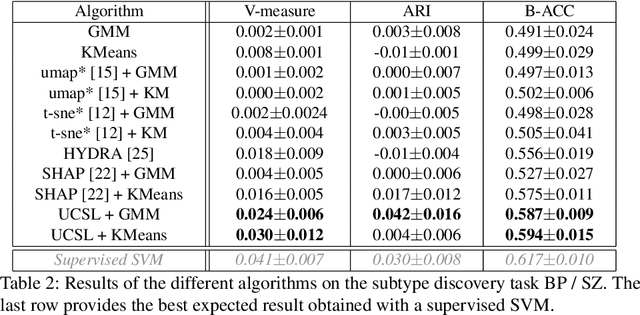
Abstract:Subtype Discovery consists in finding interpretable and consistent sub-parts of a dataset, which are also relevant to a certain supervised task. From a mathematical point of view, this can be defined as a clustering task driven by supervised learning in order to uncover subgroups in line with the supervised prediction. In this paper, we propose a general Expectation-Maximization ensemble framework entitled UCSL (Unsupervised Clustering driven by Supervised Learning). Our method is generic, it can integrate any clustering method and can be driven by both binary classification and regression. We propose to construct a non-linear model by merging multiple linear estimators, one per cluster. Each hyperplane is estimated so that it correctly discriminates - or predict - only one cluster. We use SVC or Logistic Regression for classification and SVR for regression. Furthermore, to perform cluster analysis within a more suitable space, we also propose a dimension-reduction algorithm that projects the data onto an orthonormal space relevant to the supervised task. We analyze the robustness and generalization capability of our algorithm using synthetic and experimental datasets. In particular, we validate its ability to identify suitable consistent sub-types by conducting a psychiatric-diseases cluster analysis with known ground-truth labels. The gain of the proposed method over previous state-of-the-art techniques is about +1.9 points in terms of balanced accuracy. Finally, we make codes and examples available in a scikit-learn-compatible Python package at https://github.com/neurospin-projects/2021_rlouiset_ucsl
* ECML/PKDD 2021
Contrastive Learning with Continuous Proxy Meta-Data for 3D MRI Classification
Jun 16, 2021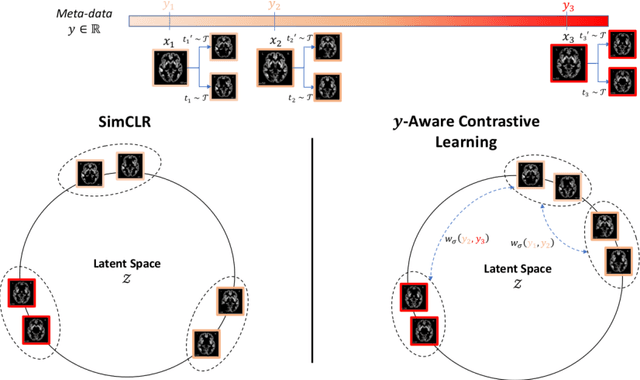
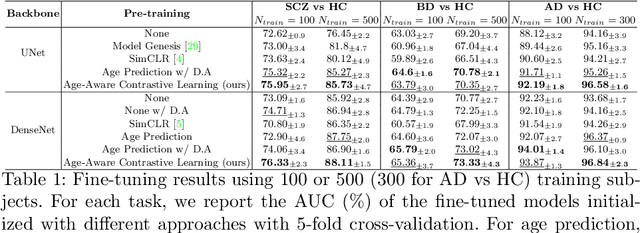
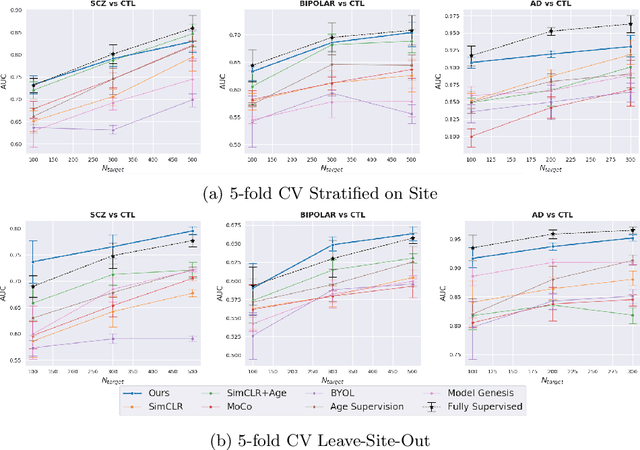
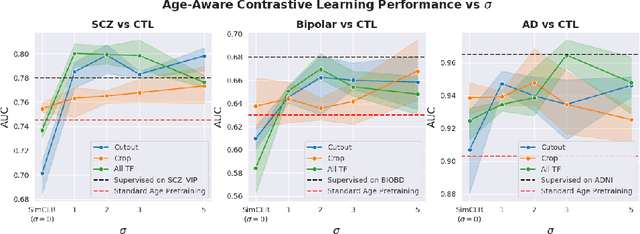
Abstract:Traditional supervised learning with deep neural networks requires a tremendous amount of labelled data to converge to a good solution. For 3D medical images, it is often impractical to build a large homogeneous annotated dataset for a specific pathology. Self-supervised methods offer a new way to learn a representation of the images in an unsupervised manner with a neural network. In particular, contrastive learning has shown great promises by (almost) matching the performance of fully-supervised CNN on vision tasks. Nonetheless, this method does not take advantage of available meta-data, such as participant's age, viewed as prior knowledge. Here, we propose to leverage continuous proxy metadata, in the contrastive learning framework, by introducing a new loss called y-Aware InfoNCE loss. Specifically, we improve the positive sampling during pre-training by adding more positive examples with similar proxy meta-data with the anchor, assuming they share similar discriminative semantic features.With our method, a 3D CNN model pre-trained on $10^4$ multi-site healthy brain MRI scans can extract relevant features for three classification tasks: schizophrenia, bipolar diagnosis and Alzheimer's detection. When fine-tuned, it also outperforms 3D CNN trained from scratch on these tasks, as well as state-of-the-art self-supervised methods. Our code is made publicly available here.
* MICCAI 2021
Benchmarking CNN on 3D Anatomical Brain MRI: Architectures, Data Augmentation and Deep Ensemble Learning
Jun 02, 2021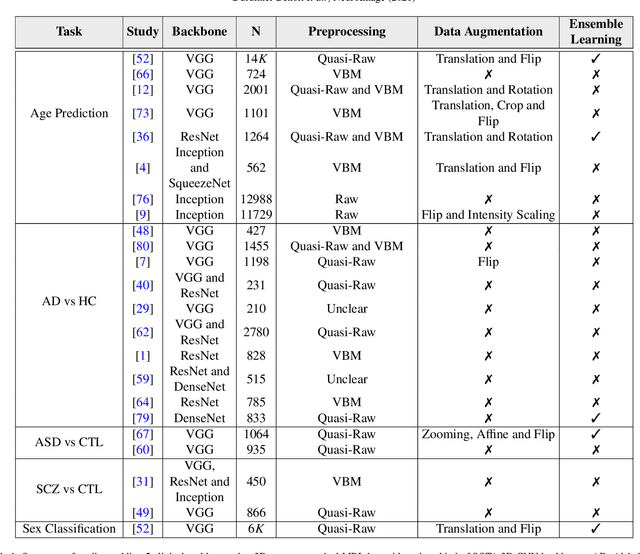
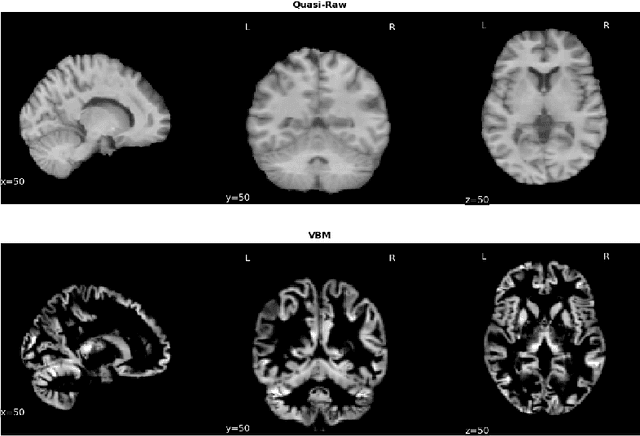
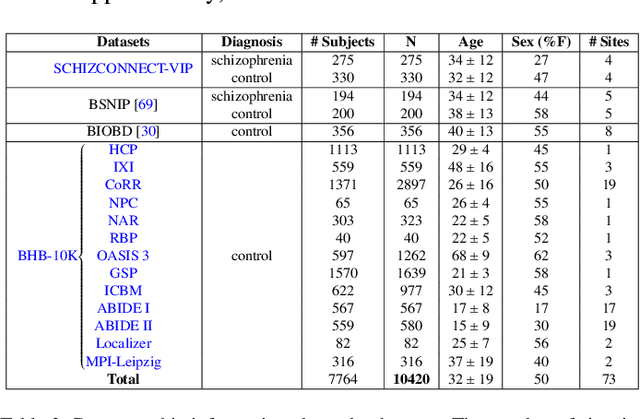
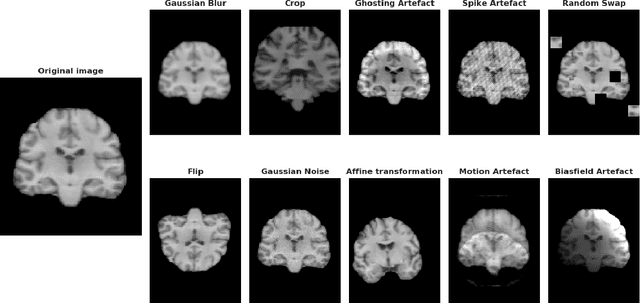
Abstract:Deep Learning (DL) and specifically CNN models have become a de facto method for a wide range of vision tasks, outperforming traditional machine learning (ML) methods. Consequently, they drew a lot of attention in the neuroimaging field in particular for phenotype prediction or computer-aided diagnosis. However, most of the current studies often deal with small single-site cohorts, along with a specific pre-processing pipeline and custom CNN architectures, which make them difficult to compare to. We propose an extensive benchmark of recent state-of-the-art (SOTA) 3D CNN, evaluating also the benefits of data augmentation and deep ensemble learning, on both Voxel-Based Morphometry (VBM) pre-processing and quasi-raw images. Experiments were conducted on a large multi-site 3D brain anatomical MRI data-set comprising N=10k scans on 3 challenging tasks: age prediction, sex classification, and schizophrenia diagnosis. We found that all models provide significantly better predictions with VBM images than quasi-raw data. This finding evolved as the training set approaches 10k samples where quasi-raw data almost reach the performance of VBM. Moreover, we showed that linear models perform comparably with SOTA CNN on VBM data. We also demonstrated that DenseNet and tiny-DenseNet, a lighter version that we proposed, provide a good compromise in terms of performance in all data regime. Therefore, we suggest to employ them as the architectures by default. Critically, we also showed that current CNN are still very biased towards the acquisition site, even when trained with N=10k multi-site images. In this context, VBM pre-processing provides an efficient way to limit this site effect. Surprisingly, we did not find any clear benefit from data augmentation techniques. Finally, we proved that deep ensemble learning is well suited to re-calibrate big CNN models without sacrificing performance.
Continuation of Nesterov's Smoothing for Regression with Structured Sparsity in High-Dimensional Neuroimaging
Apr 22, 2018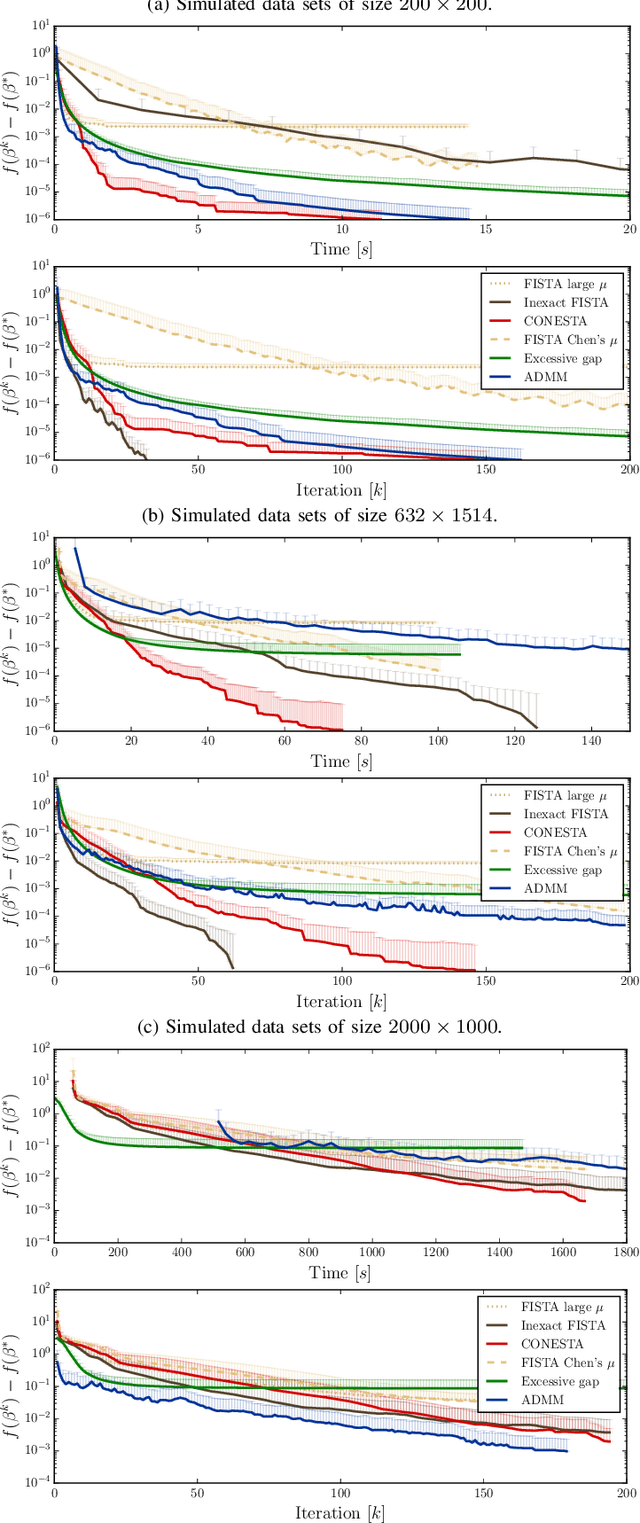
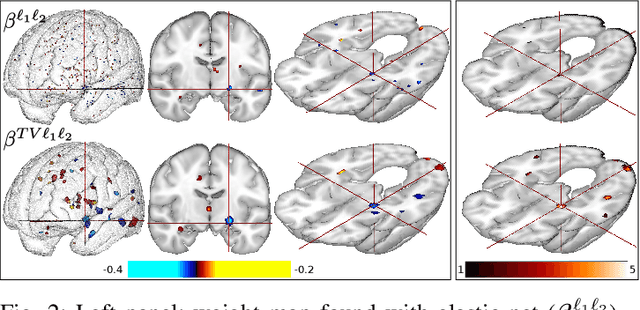
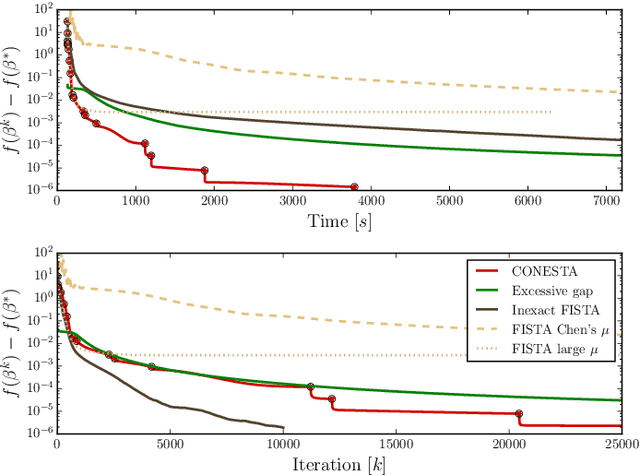
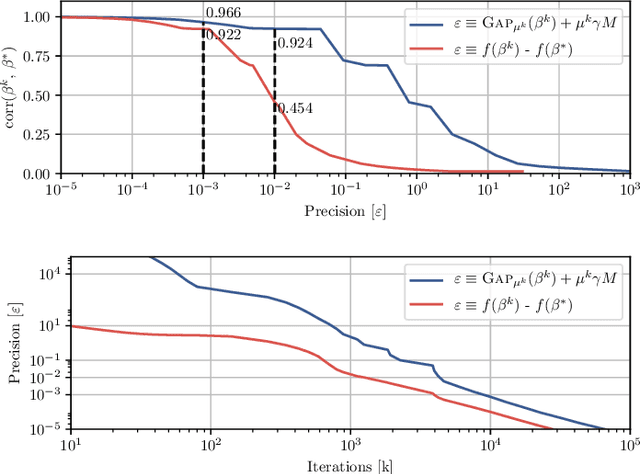
Abstract:Predictive models can be used on high-dimensional brain images for diagnosis of a clinical condition. Spatial regularization through structured sparsity offers new perspectives in this context and reduces the risk of overfitting the model while providing interpretable neuroimaging signatures by forcing the solution to adhere to domain-specific constraints. Total Variation (TV) enforces spatial smoothness of the solution while segmenting predictive regions from the background. We consider the problem of minimizing the sum of a smooth convex loss, a non-smooth convex penalty (whose proximal operator is known) and a wide range of possible complex, non-smooth convex structured penalties such as TV or overlapping group Lasso. Existing solvers are either limited in the functions they can minimize or in their practical capacity to scale to high-dimensional imaging data. Nesterov's smoothing technique can be used to minimize a large number of non-smooth convex structured penalties but reasonable precision requires a small smoothing parameter, which slows down the convergence speed. To benefit from the versatility of Nesterov's smoothing technique, we propose a first order continuation algorithm, CONESTA, which automatically generates a sequence of decreasing smoothing parameters. The generated sequence maintains the optimal convergence speed towards any globally desired precision. Our main contributions are: To propose an expression of the duality gap to probe the current distance to the global optimum in order to adapt the smoothing parameter and the convergence speed. We provide a convergence rate, which is an improvement over classical proximal gradient smoothing methods. We demonstrate on both simulated and high-dimensional structural neuroimaging data that CONESTA significantly outperforms many state-of-the-art solvers in regard to convergence speed and precision.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge