Damon Wing Kee Wong
Angle-Closure Detection in Anterior Segment OCT based on Multi-Level Deep Network
Feb 10, 2019



Abstract:Irreversible visual impairment is often caused by primary angle-closure glaucoma, which could be detected via Anterior Segment Optical Coherence Tomography (AS-OCT). In this paper, an automated system based on deep learning is presented for angle-closure detection in AS-OCT images. Our system learns a discriminative representation from training data that captures subtle visual cues not modeled by handcrafted features. A Multi-Level Deep Network (MLDN) is proposed to formulate this learning, which utilizes three particular AS-OCT regions based on clinical priors: the global anterior segment structure, local iris region, and anterior chamber angle (ACA) patch. In our method, a sliding window based detector is designed to localize the ACA region, which addresses ACA detection as a regression task. Then, three parallel sub-networks are applied to extract AS-OCT representations for the global image and at clinically-relevant local regions. Finally, the extracted deep features of these sub-networks are concatenated into one fully connected layer to predict the angle-closure detection result. In the experiments, our system is shown to surpass previous detection methods and other deep learning systems on two clinical AS-OCT datasets.
Multi-Context Deep Network for Angle-Closure Glaucoma Screening in Anterior Segment OCT
Sep 10, 2018



Abstract:A major cause of irreversible visual impairment is angle-closure glaucoma, which can be screened through imagery from Anterior Segment Optical Coherence Tomography (AS-OCT). Previous computational diagnostic techniques address this screening problem by extracting specific clinical measurements or handcrafted visual features from the images for classification. In this paper, we instead propose to learn from training data a discriminative representation that may capture subtle visual cues not modeled by predefined features. Based on clinical priors, we formulate this learning with a presented Multi-Context Deep Network (MCDN) architecture, in which parallel Convolutional Neural Networks are applied to particular image regions and at corresponding scales known to be informative for clinically diagnosing angle-closure glaucoma. The output feature maps of the parallel streams are merged into a classification layer to produce the deep screening result. Moreover, we incorporate estimated clinical parameters to further enhance performance. On a clinical AS-OCT dataset, our system is validated through comparisons to previous screening methods.
Structure-preserving Guided Retinal Image Filtering and Its Application for Optic Disc Analysis
May 22, 2018



Abstract:Retinal fundus photographs have been used in the diagnosis of many ocular diseases such as glaucoma, pathological myopia, age-related macular degeneration and diabetic retinopathy. With the development of computer science, computer aided diagnosis has been developed to process and analyse the retinal images automatically. One of the challenges in the analysis is that the quality of the retinal image is often degraded. For example, a cataract in human lens will attenuate the retinal image, just as a cloudy camera lens which reduces the quality of a photograph. It often obscures the details in the retinal images and posts challenges in retinal image processing and analysing tasks. In this paper, we approximate the degradation of the retinal images as a combination of human-lens attenuation and scattering. A novel structure-preserving guided retinal image filtering (SGRIF) is then proposed to restore images based on the attenuation and scattering model. The proposed SGRIF consists of a step of global structure transferring and a step of global edge-preserving smoothing. Our results show that the proposed SGRIF method is able to improve the contrast of retinal images, measured by histogram flatness measure, histogram spread and variability of local luminosity. In addition, we further explored the benefits of SGRIF for subsequent retinal image processing and analysing tasks. In the two applications of deep learning based optic cup segmentation and sparse learning based cup-to-disc ratio (CDR) computation, our results show that we are able to achieve more accurate optic cup segmentation and CDR measurements from images processed by SGRIF.
* Accepted for publication on IEEE Trans. on Medical Imaging
Disc-aware Ensemble Network for Glaucoma Screening from Fundus Image
May 19, 2018


Abstract:Glaucoma is a chronic eye disease that leads to irreversible vision loss. Most of the existing automatic screening methods firstly segment the main structure, and subsequently calculate the clinical measurement for detection and screening of glaucoma. However, these measurement-based methods rely heavily on the segmentation accuracy, and ignore various visual features. In this paper, we introduce a deep learning technique to gain additional image-relevant information, and screen glaucoma from the fundus image directly. Specifically, a novel Disc-aware Ensemble Network (DENet) for automatic glaucoma screening is proposed, which integrates the deep hierarchical context of the global fundus image and the local optic disc region. Four deep streams on different levels and modules are respectively considered as global image stream, segmentation-guided network, local disc region stream, and disc polar transformation stream. Finally, the output probabilities of different streams are fused as the final screening result. The experiments on two glaucoma datasets (SCES and new SINDI datasets) show our method outperforms other state-of-the-art algorithms.
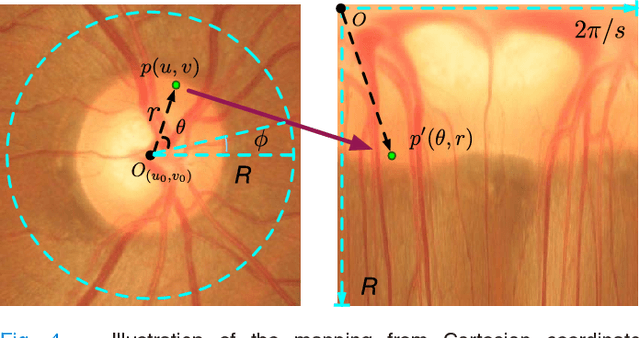
Joint Optic Disc and Cup Segmentation Based on Multi-label Deep Network and Polar Transformation
Jan 11, 2018



Abstract:Glaucoma is a chronic eye disease that leads to irreversible vision loss. The cup to disc ratio (CDR) plays an important role in the screening and diagnosis of glaucoma. Thus, the accurate and automatic segmentation of optic disc (OD) and optic cup (OC) from fundus images is a fundamental task. Most existing methods segment them separately, and rely on hand-crafted visual feature from fundus images. In this paper, we propose a deep learning architecture, named M-Net, which solves the OD and OC segmentation jointly in a one-stage multi-label system. The proposed M-Net mainly consists of multi-scale input layer, U-shape convolutional network, side-output layer, and multi-label loss function. The multi-scale input layer constructs an image pyramid to achieve multiple level receptive field sizes. The U-shape convolutional network is employed as the main body network structure to learn the rich hierarchical representation, while the side-output layer acts as an early classifier that produces a companion local prediction map for different scale layers. Finally, a multi-label loss function is proposed to generate the final segmentation map. For improving the segmentation performance further, we also introduce the polar transformation, which provides the representation of the original image in the polar coordinate system. The experiments show that our M-Net system achieves state-of-the-art OD and OC segmentation result on ORIGA dataset. Simultaneously, the proposed method also obtains the satisfactory glaucoma screening performances with calculated CDR value on both ORIGA and SCES datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge